Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope
- PMID: 10066836
- PMCID: PMC98962
- DOI: 10.1128/MMBR.63.1.174-229.1999
Surface proteins of gram-positive bacteria and mechanisms of their targeting to the cell wall envelope
Abstract
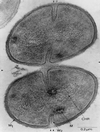
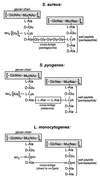
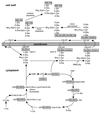
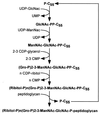
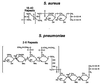
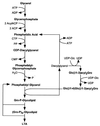
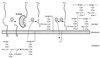
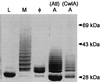
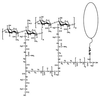
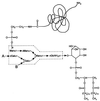
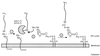
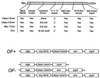
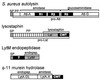
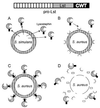
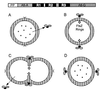
The cell wall envelope of gram-positive bacteria is a macromolecular, exoskeletal organelle that is assembled and turned over at designated sites. The cell wall also functions as a surface organelle that allows gram-positive pathogens to interact with their environment, in particular the tissues of the infected host. All of these functions require that surface proteins and enzymes be properly targeted to the cell wall envelope. Two basic mechanisms, cell wall sorting and targeting, have been identified. Cell well sorting is the covalent attachment of surface proteins to the peptidoglycan via a C-terminal sorting signal that contains a consensus LPXTG sequence. More than 100 proteins that possess cell wall-sorting signals, including the M proteins of Streptococcus pyogenes, protein A of Staphylococcus aureus, and several internalins of Listeria monocytogenes, have been identified. Cell wall targeting involves the noncovalent attachment of proteins to the cell surface via specialized binding domains. Several of these wall-binding domains appear to interact with secondary wall polymers that are associated with the peptidoglycan, for example teichoic acids and polysaccharides. Proteins that are targeted to the cell surface include muralytic enzymes such as autolysins, lysostaphin, and phage lytic enzymes. Other examples for targeted proteins are the surface S-layer proteins of bacilli and clostridia, as well as virulence factors required for the pathogenesis of L. monocytogenes (internalin B) and Streptococcus pneumoniae (PspA) infections. In this review we describe the mechanisms for both sorting and targeting of proteins to the envelope of gram-positive bacteria and review the functions of known surface proteins.
Figures





References
-
- Adler L A, Arvidson S. Correlation between the rate of exoprotein synthesis and the amount of the multiprotein complex on membrane-bound ribosomes (MBRP-complex) in Staphylococcus aureus. J Gen Microbiol. 1987;133:803–813. - PubMed
-
- Åkerström B, Björck L. Protein L: an immunoglobulin light chain-binding bacterial protein. Characterization of binding and physicochemical properties. J Biol Chem. 1989;264:19740–19746. - PubMed
-
- Åkerström B, Lindqvist A, Lindahl G. Binding properties of protein Arp, a bacterial IgA-receptor. Mol Immunol. 1991;28:349–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

