Effects of tetracaine on sarcoplasmic calcium release in mammalian skeletal muscle fibres
- PMID: 10066909
- PMCID: PMC2269186
- DOI: 10.1111/j.1469-7793.1999.843ab.x
Effects of tetracaine on sarcoplasmic calcium release in mammalian skeletal muscle fibres
Abstract
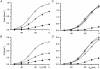
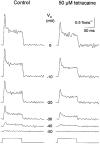
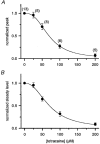
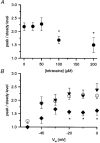
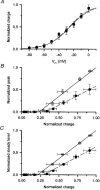
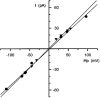
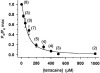
1. Single muscle fibres were dissociated enzymatically from the extensor digitorum communis muscle of rats. The fibres were mounted into a double Vaseline gap experimental chamber and the events in excitation-contraction coupling were studied under voltage clamp conditions in the presence and absence of the local anaesthetic tetracaine. 2. Changes in intracellular calcium concentration ([Ca2+]i) were monitored using the calcium sensitive dyes antipyrylazo III and fura-2 and the rate of calcium release (Rrel) from the sarcoplasmic reticulum (SR) was calculated. Tetracaine decreased the maximal attained [Ca2+]i and suppressed, in a dose-dependent manner, both the early peak and the steady level of Rrel in the voltage range examined. 3. The concentration dependence of the effects on the two kinetic components of Rrel were almost identical with a half-effective concentration (K50) of 70 and 71 microM and a Hill coefficient (nH) of 2.7 and 2.3 for the peak and the steady level, respectively. Furthermore, the drug did not alter the peak to steady level ratio up to a concentration (50 microM) that caused a 35 +/- 5 % reduction in calcium release. Higher concentrations did suppress the ratio but the degree of suppression was voltage independent. 4. Tetracaine (50 microM) neither influenced the total available intramembrane charge nor altered its membrane potential dependence. It shifted the transfer function, the normalized SR permeability versus normalized charge to the right, indicating that similar charge transfer caused a smaller increase in SR permeability. 5. To explore the site of action of tetracaine further the ryanodine receptor (RyR) calcium release channel of the SR was purified and reconstituted into planar lipid bilayers. The reconstituted channel had a conductance of 511 +/- 14 pS (n = 8) in symmetric 250 mM KCl that was not affected by tetracaine. Tetracaine decreased the open probability of the channel in a concentration-dependent manner with K50 = 68 microM and nH = 1.5. 6. These experiments show that tetracaine suppresses SR calcium release in enzymatic isolated mammalian skeletal muscle fibres. This effect is due, presumably, to the decreased open probability of the RyR in the presence of the drug. Since both the inactivating peak and the steady level of Rrel were equally affected by tetracaine, our observations suggest that there is a tight coupling between these kinetic components of SR calcium release in mammalian skeletal muscle.
Figures




References
-
- Amirsén R, Sierralta J, Vélez P, Naranjo D, Suárez-Isla BA. Modal gating in neuronal and skeletal muscle ryanodine-sensitive Ca2+ release channels. American Journal of Physiology. 1996;271:C144–153. - PubMed
-
- Bianchi CP, Bolton TC. Action of local anaesthetics on coupling systems in muscle. Journal of Pharmacology and Experimental Therapeutics. 1967;157:388–405. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

