Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death
- PMID: 10066918
- PMCID: PMC2269224
- DOI: 10.1111/j.1469-7793.1999.001aa.x
Contributions of mitochondria to animal physiology: from homeostatic sensor to calcium signalling and cell death
Abstract
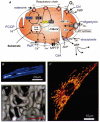
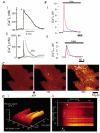
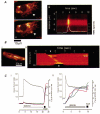
Over recent years, it has become clear that mitochondria play a central role in many key aspects of animal physiology and pathophysiology. Their central and ubiquitous task is clearly the production of ATP. Nevertheless, they also play subtle roles in glucose homeostasis, acting as the sensor for substrate supply in the transduction pathway that promotes insulin secretion by the pancreatic -cell and that modulates the excitability of the hypothalamic glucose-sensitive neurons involved in appetite control. Mitochondria may also act as sensors of availability of oxygen, the other major mitochondrial substrate, in the regulation of respiration. Mitochondria take up calcium, and the high opacity mitochondrial calcium uptake pathway provides a mechanism that couples energy demand to increased ATP production through the calcium-dependent upregulation of mitochondrial enzyme activity. Mitochondrial calcium accumulation may also have a substantial impact on the spatiotemporal dynamics of cellular calcium signals, with subtle differences of detail in different cell types. Recent work has also revealed the centrality of mitochondrial dysfunction as an irreversible step in the pathway to both necrotic and apoptotic cell death. This review looks at recent developments in these rapidly evolving areas of cell physiology in an attempt to draw together disparate areas of research into a common theme.
Figures


References
-
- Acker H. Oxygen sensing in the carotid body: ideas and models. Advances in Experimental Medicine and Biology. 1994;360:21–27. - PubMed
-
- Anichkov SV, Belen'kii ML. Pharmacology of the Carotid Body Chemo-receptors. New York: MacMillan; 1963.
-
- Ashcroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends in Neurosciences. 1998;21:288–294. - PubMed
-
- Ashcroft FM, Rorsman P. ATP-sensitive K+ channels: a link between B-cell metabolism and insulin secretion. Biochemical Society Transactions. 1989;18:109–111. - PubMed
-
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflügers Archiv. 1990;415:479–483. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

