Feedback inhibition of rat amiloride-sensitive epithelial sodium channels expressed in Xenopus laevis oocytes
- PMID: 10066920
- PMCID: PMC2269211
- DOI: 10.1111/j.1469-7793.1999.031aa.x
Feedback inhibition of rat amiloride-sensitive epithelial sodium channels expressed in Xenopus laevis oocytes
Abstract
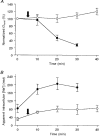
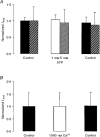
1. Regulation of the amiloride-sensitive epithelial sodium channel (ENaC) is essential for the control of body sodium homeostasis. The downregulation of the activity of this Na+ channel that occurs when the intracellular Na+ concentration ([Na+]i) is increased is known as feedback inhibition. Although intracellular Na+ is the trigger for this phenomenon, its cellular and molecular mediators are unknown. 2. We used the 'cut-open oocyte' technique to control the composition of the intracellular milieu of Xenopus oocytes expressing rat ENaCs to enable us to test several factors potentially involved in feedback inhibition. 3. The effects of perfusion of the intracellular space were demonstrated by an electromicrographic study and the time course of the intracellular solution exchange was established by observing the effect of intracellular pH: a decrease from pH 7.4 to 6.5 reduced the amiloride-sensitive current by about 40 % within 2 min. 4. Feedback inhibition was observed in non-perfused oocytes when Na+ entry induced a large increase in [Na+]i. Intracellular perfusion prevented feedback regulation even though the [Na+]i was allowed to increase to values above 50 mM. 5. No effects on the amiloride-sensitive current were observed after changes in the concentration of Na+ (from 1 to 50 mM), Ca2+ (from 10 to 1000 nM) or ATP (from nominally free to 1 or 5 mM) in the intracellular perfusate. 6. We conclude that feedback inhibition requires intracellular factors that can be removed by intracellular perfusion. Although a rise in [Na+]i may be the trigger for the feedback inhibition of the ENaC, this effect is not mediated by a direct effect of Na+, Ca2+ or ATP on the ENaC protein.
Figures

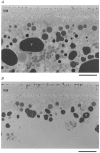
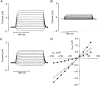
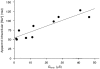
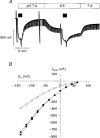

References
-
- Benos DJ, Fuller CM, Shlyonsky VG, Berdiev BK, Ismailov II. Amiloride-sensitive Na+ channels - insights and outlooks. News in Physiological Sciences. 1997;12:55–61.
-
- Carmeliet E. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells? Cardiovascular Research. 1992;26:433–442. - PubMed
-
- Chang D, Hsieh PS, Dawson DC. Calcium: a program in BASIC for calculating the composition of solutions with specified free concentrations of calcium, magnesium and other divalent cations. Computers in Biology & Medicine. 1988;18:351–366. - PubMed
-
- Chang SS, Grunder S, Hanukoglu A, Rosler A, Mathew PM, Hanukoglu I, Schild L, Lu Y, Shimkets RA, Nelson-Williams C, Rossier BC, Lifton RP. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nature Genetics. 1996;12:248–253. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

