The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones
- PMID: 10066931
- PMCID: PMC2269213
- DOI: 10.1111/j.1469-7793.1999.163aa.x
The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones
Abstract
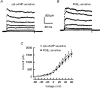
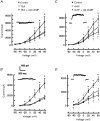
1. The role of the cyclic AMP (cAMP) transduction cascade in mediating the prostaglandin E2 (PGE2)-induced decrease in potassium current (IK) was investigated in isolated embryonic rat sensory neurones using the whole-cell patch-clamp recording technique. 2. Exposure to 100 microM chlorophenylthio-adenosine cyclic 3', 5'-monophosphate (cpt-cAMP) or 1 microM PGE2 caused a slow suppression of the whole-cell IK by 34 and 36 %, respectively (measured after 20 min), without a shift in the voltage dependence of activation for this current. Neither of these agents altered the shape of the voltage-dependent inactivation curve indicating that the suppression of IK did not result from alterations in the inactivation properties. 3. To determine whether the PGE2-mediated suppression of IK depended on activation of the cAMP pathway, cells were exposed to this prostanoid in the presence of the protein kinase A (PKA) inhibitor, PKI. The PGE2-induced suppression of IK was prevented by PKI. In the absence of PGE2, PKI had no significant effect on the magnitude of IK. 4. Results obtained from protocols using different conditioning prepulse voltages indicated that the extent of cpt-cAMP- and PGE2-mediated suppression of IK was independent of the prepulse voltage. The subtraction of control and treated currents revealed that the cpt-cAMP- and PGE2-sensitive currents exhibited little time-dependent inactivation. Taken together, these results suggest that the modulated currents may be delayed rectifier-like IK. 5. Exposure to the inhibitors of IK, tetraethylammonium (TEA) or 4-aminopyridine (4-AP), reduced the control current elicited by a voltage step to +60 mV by 40-50 %. In the presence of 10 mM TEA, treatment with cpt-cAMP did not result in any further inhibition of IK. In contrast, cpt-cAMP reduced IK by an additional 25-30 % in the presence of 1 mM 4-AP. This effect was independent of the conditioning prepulse voltage. 6. These results establish that PGE2 inhibits an outward IK in sensory neurones via activation of PKA and are consistent with the idea that the PGE2-mediated sensitization of sensory neurones results, in part, from an inhibition of delayed rectifier-like IK.
Figures








References
-
- Akins PT, McCleskey EW. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience. 1993;56:759–769. 10.1016/0306-4522(93)90372-M. - DOI - PubMed
-
- Alloatti G, Serazzi L, Levi RC. Prostaglandin I2 (PGI2) enhances calcium current in guinea-pig ventricular heart cells. Journal of Molecular and Cellular Cardiology. 1991;23:851–860. 10.1016/0022-2828(91)90218-B. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

