Quantal amplitude and quantal variance of strontium-induced asynchronous EPSCs in rat dentate granule neurons
- PMID: 10066937
- PMCID: PMC2269216
- DOI: 10.1111/j.1469-7793.1999.227aa.x
Quantal amplitude and quantal variance of strontium-induced asynchronous EPSCs in rat dentate granule neurons
Abstract
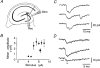
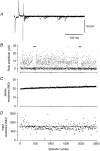
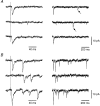
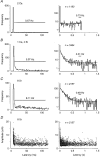
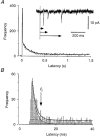
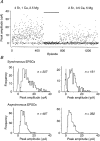
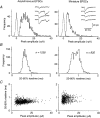
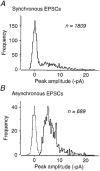
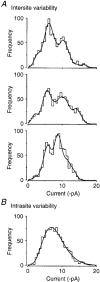
1. Excitatory postsynaptic currents (EPSCs) were recorded from granule cells of the dentate gyrus in acute slices of 17- to 21-day-old rats (22-25 C) using tissue cuts and minimal extracellular stimulation to selectively activate a small number of synaptic contacts. 2. Adding millimolar Sr2+ to the external solution produced asynchronous EPSCs (aEPSCs) lasting for several hundred milliseconds after the stimulus. Minimally stimulated aEPSCs resembled miniature EPSCs (mEPSCs) recorded in the same cell but differed from them in ways expected from the greater range of dendritic filtering experienced by mEPSCs. aEPSCs had the same stimulus threshold as the synchronous EPSCs (sEPSCs) that followed the stimulus with a brief latency. aEPSCs following stimulation of distal inputs had a slower mean rise time than those following stimulation of proximal inputs. These results suggest that aEPSCs arose from the same synapses that generated sEPSCs. 3. Proximally elicited aEPSCs had a mean amplitude of 6.7 +/- 2.2 pA (+/- s.d., n = 23 cells) at -70 mV and an amplitude coefficient of variation of 0. 46 +/- 0.08. 4. The amplitude distributions of sEPSCs never exhibited distinct peaks. 5. Monte Carlo modelling of the shapes of aEPSC amplitude distributions indicated that our data were best explained by an intrasite model of quantal variance. 6. It is concluded that Sr2+-evoked aEPSCs are uniquantal events arising at synaptic terminals that were recently invaded by an action potential, and so provide direct information about the quantal amplitude and quantal variance at those terminals. The large quantal variance obscures quantization of the amplitudes of evoked sEPSCs at this class of excitatory synapse.
Figures




References
-
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. In: Storm-Mathison J, Zimmer J, Ottersen OP, editors. Progress in Brain Research. New York: Elsevier; 1990. pp. 1–11. - PubMed
-
- Bekkers JM. Quantal analysis of synaptic transmission in the central nervous system. Current Opinion in Neurobiology. 1994;4:360–365. 10.1016/0959-4388(94)90097-3. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

