Nitric oxide release in penile corpora cavernosa in a rat model of erection
- PMID: 10066939
- PMCID: PMC2269210
- DOI: 10.1111/j.1469-7793.1999.261aa.x
Nitric oxide release in penile corpora cavernosa in a rat model of erection
Abstract
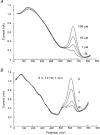
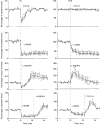
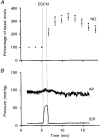
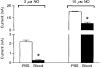
1. Nitric oxide (NO) levels were measured in the corpus cavernosum of urethane-anaesthetized rats by using differential normal pulse voltammetry with carbon fibre microelectrodes coated with a polymeric porphyrin and a cation exchanger (Nafion). A NO oxidation peak could be recorded at 650 mV vs. a Ag-AgCl reference electrode every 100 s. 2. This NO signal was greatly decreased by the NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME), given by local and systemic routes, and enhanced by the NO precursor L-arginine. Treatment with L-arginine reversed the effect of L-NAME on the NO peak. 3. Both the NO signal and the intracavernosal pressure (ICP) were increased by electrical stimulation of cavernosal nerves (ESCN). However, the rise in the NO levels long outlived the rapid return to baseline of the ICP values at the end of nerve stimulation. 4. The ICP and the NO responses to ESCN were suppressed by local and systemic injections of L-NAME. Subsequent treatment with L-arginine of L-NAME-treated animals restored the NO signal to basal levels and the NO response to ESCN. The ICP response to ESCN was restored only in part by L-arginine. 5. The observed temporal dissociation between the NO and ICP responses could be accounted for by several factors, including the buffering of NO by the blood filling the cavernosal spaces during erection. 6. These findings indicate that an increased production of NO in the corpora cavernosa is necessary but not sufficient for maintaining penile erection and suggest a complex modulation of the NO-cGMP-cavernosal smooth muscle relaxation cascade.
Figures


Similar articles
-
Nitric oxide as a mediator of cocaine-induced penile erection in the rat.Br J Pharmacol. 1996 May;118(1):155-61. doi: 10.1111/j.1476-5381.1996.tb15379.x. Br J Pharmacol. 1996. PMID: 8733589 Free PMC article.
-
Involvement of L-arginine/nitric oxide pathway at the paraventricular nucleus of hypothalamus in central neural regulation of penile erection in the rat.Int J Impot Res. 2002 Jun;14(3):139-45. doi: 10.1038/sj.ijir.3900825. Int J Impot Res. 2002. PMID: 12058240
-
In vivo electrochemical measurement of nitric oxide in corpus cavernosum penis.J Neurosci Methods. 2002 Sep 30;119(2):143-50. doi: 10.1016/s0165-0270(02)00173-5. J Neurosci Methods. 2002. PMID: 12323418
-
Regulation of pre-synaptic alpha adrenergic activity in the corpus cavernosum.Int J Impot Res. 2000 Mar;12 Suppl 1:S20-25. doi: 10.1038/sj.ijir.3900500. Int J Impot Res. 2000. PMID: 10845761 Review.
-
The pleiotropic effects of inducible nitric oxide synthase (iNOS) on the physiology and pathology of penile erection.Curr Pharm Des. 2005;11(31):4041-6. doi: 10.2174/138161205774913372. Curr Pharm Des. 2005. PMID: 16378509 Review.
Cited by
-
European Society for Sexual Medicine Consensus Statement on the Use of the Cavernous Nerve Injury Rodent Model to Study Postradical Prostatectomy Erectile Dysfunction.Sex Med. 2020 Sep;8(3):327-337. doi: 10.1016/j.esxm.2020.06.007. Epub 2020 Jul 13. Sex Med. 2020. PMID: 32674971 Free PMC article. Review.
-
Proniosomal Gel-Loaded Phosphodiesterase Inhibitors (Sildenafil, Vardenafil, and Tadalafil): Prospects for Topical Penile Therapy of Tadalafil for Treatment of Erectile Dysfunction.Gels. 2023 Jul 25;9(8):597. doi: 10.3390/gels9080597. Gels. 2023. PMID: 37623052 Free PMC article.
-
Ex vivo relaxation effect of Cuscuta chinensis extract on rabbit corpus cavernosum.Asian J Androl. 2013 Jan;15(1):134-7. doi: 10.1038/aja.2012.124. Epub 2012 Nov 12. Asian J Androl. 2013. PMID: 23147465 Free PMC article.
-
The Cavernous Nerve Injury Rat Model: A Pictorial Essay on Post-Radical Prostatectomy Erectile Dysfunction Research.Life (Basel). 2023 Dec 13;13(12):2337. doi: 10.3390/life13122337. Life (Basel). 2023. PMID: 38137938 Free PMC article. Review.
-
Nitric oxide-mediated cortical activation: a diffuse wake-up system.J Neurosci. 2003 May 15;23(10):4299-307. doi: 10.1523/JNEUROSCI.23-10-04299.2003. J Neurosci. 2003. PMID: 12764118 Free PMC article.
References
-
- Archer S. Measurement of nitric oxide in biological models. FASEB Journal. 1993;7:349–360. - PubMed
-
- Boolell M, Allan MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingel C. Sildenafil: an oral active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. International Journal of Impotence Research. 1996;8:47–52. - PubMed
-
- Burlet S, Cespuglio R. Voltammetric detection of nitric oxide (NO) in the rat brain: its variations throughout the sleep-wake cycle. Neuroscience Letters. 1997;226:131–135. - PubMed
-
- Burnett AL. Role of nitric oxide in the physiology of erection. Biology of Reproduction. 1995;52:485–489. - PubMed
-
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TSK, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

