Respiratory and cardiac modulation of single sympathetic vasoconstrictor and sudomotor neurones to human skin
- PMID: 10066943
- PMCID: PMC2269223
- DOI: 10.1111/j.1469-7793.1999.303aa.x
Respiratory and cardiac modulation of single sympathetic vasoconstrictor and sudomotor neurones to human skin
Abstract
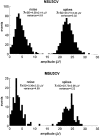
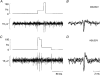
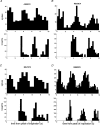
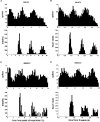
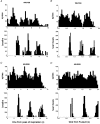
1. The firing of single sympathetic neurones was recorded via tungsten microelectrodes in cutaneous fascicles of the peroneal nerve in awake humans. Studies were made of 17 vasoconstrictor neurones during cold-induced cutaneous vasoconstriction and eight sudomotor neurones during heat-induced sweating. Oligounitary recordings were obtained from 8 cutaneous vasconstrictor and 10 sudomotor sites. Skin blood flow was measured by laser Doppler flowmetry, and sweating by changes in skin electrical resistance within the innervation territory on the dorsum of the foot. 2. Perispike time histograms revealed respiratory modulation in 11 (65 %) vasoconstrictor and 4 (50 %) sudomotor neurones. After correcting for estimated conduction delays, the firing probability was higher in inspiration for both classes of neurone. Measured from the oligounitary recordings, the respiratory modulation indices were 67. 7 +/- 3.9 % for vasoconstrictor and 73.5 +/- 5.7 % for sudomotor neurones (means +/- s.e.m.). As previously found for sudomotor neurones, cardiac rhythmicity was expressed by 7 (41 %) vasoconstrictor neurones, 5 of which showed no significant coupling to respiration. Measured from the oligounitary records, the cardiac modulation of cutaneous vasoconstrictor activity was 58.6 +/- 4.9 %, compared with 74.4 +/- 6.4 % for sudomotor activity. 3. Both vasoconstrictor and sudomotor neurones displayed low average firing rates (0.53 and 0.62 Hz, respectively). The percentage of cardiac intervals in which units fired was 38 % and 35 %, respectively. Moreover, when considering only those cardiac intervals when a unit fired, vasoconstrictor and sudomotor neurones generated a single spike 66 % and 67 % of the time. Rarely were more than four spikes generated by a single neurone. 4. We conclude that human cutaneous vasoconstrictor and sudomotor neurones share several properties: both classes contain subpopulations that are modulated by respiration and/or the cardiac cycle. The data suggest that the intensity of a multi-unit burst of vasoconstrictor or sudomotor impulses is probably governed primarily by firing incidence and the recruitment of additional neurones, rather than by an increase in the number of spikes each unit contributes to a burst.
Figures




Similar articles
-
The discharge behaviour of single sympathetic neurones supplying human sweat glands.J Auton Nerv Syst. 1996 Dec 14;61(3):277-86. doi: 10.1016/s0165-1838(96)00095-1. J Auton Nerv Syst. 1996. PMID: 8988486 Clinical Trial.
-
Firing properties of sudomotor neurones in hyperhidrosis and thermal sweating.Clin Auton Res. 2008 Dec;18(6):325-30. doi: 10.1007/s10286-008-0507-7. Epub 2008 Nov 6. Clin Auton Res. 2008. PMID: 18989617
-
Cardiorespiratory coupling of sympathetic outflow in humans: a comparison of respiratory and cardiac modulation of sympathetic nerve activity to skin and muscle.Exp Physiol. 2013 Sep;98(9):1327-36. doi: 10.1113/expphysiol.2013.072421. Epub 2013 Apr 26. Exp Physiol. 2013. PMID: 23625953
-
Why do human postganglionic neurones primarily only fire once during a sympathetic burst?Acta Physiol Scand. 2003 Mar;177(3):247-53. doi: 10.1046/j.1365-201X.2003.01078.x. Acta Physiol Scand. 2003. PMID: 12608995 Review.
-
Coordination of sympathetic and respiratory systems: neurophysiological experiments.Clin Exp Hypertens. 1995 Jan-Feb;17(1-2):223-35. doi: 10.3109/10641969509087067. Clin Exp Hypertens. 1995. PMID: 7735271 Review.
Cited by
-
On the number of preganglionic neurons driving human postganglionic sympathetic neurons: a comparison of modeling and empirical data.Front Neurosci. 2011 Dec 6;5:132. doi: 10.3389/fnins.2011.00132. eCollection 2011. Front Neurosci. 2011. PMID: 22164130 Free PMC article.
-
Human cardiovascular responses to passive heat stress.Compr Physiol. 2015 Jan;5(1):17-43. doi: 10.1002/cphy.c140015. Compr Physiol. 2015. PMID: 25589263 Free PMC article. Review.
-
ATP overflow in skeletal muscle 1A arterioles.J Physiol. 2010 Aug 15;588(Pt 16):3089-100. doi: 10.1113/jphysiol.2010.193094. Epub 2010 Jun 21. J Physiol. 2010. PMID: 20566660 Free PMC article.
-
Human sympathetic outflows to skin and muscle target organs fluctuate concordantly over a wide range of time-varying frequencies.J Physiol. 2012 Jan 15;590(2):363-75. doi: 10.1113/jphysiol.2011.214528. Epub 2011 Nov 7. J Physiol. 2012. PMID: 22063627 Free PMC article. Clinical Trial.
-
Low-frequency physiological activation of the vestibular utricle causes biphasic modulation of skin sympathetic nerve activity in humans.Exp Brain Res. 2012 Jul;220(2):101-8. doi: 10.1007/s00221-012-3118-4. Epub 2012 May 24. Exp Brain Res. 2012. PMID: 22623094
References
-
- Andersson PO. Comparative vascular effects of stimulation continuously and in bursts of the sympathetic nerves to cat skeletal muscle. Acta Physiologica Scandinavica. 1983;118:343–348. - PubMed
-
- Bini G, Hagbarth K-E, Wallin BG. Cardiac rhythmicity of skin sympathetic activity recorded from peripheral nerves in man. Journal of the Autonomic Nervous System. 1981;4:17–24. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

