Absence of spontaneous central nervous system remyelination in class II-deficient mice infected with Theiler's virus
- PMID: 10068316
- PMCID: PMC5444470
- DOI: 10.1097/00005072-199901000-00009
Absence of spontaneous central nervous system remyelination in class II-deficient mice infected with Theiler's virus
Abstract
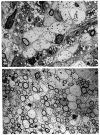
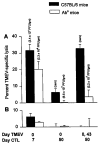
We previously showed that Theiler's murine encephalomyelitis virus (TMEV)-infected major histocompatibility complex (MHC) class II-deficient mice develop both demyelination and neurologic deficits, whereas MHC class I-deficient mice develop demyelination but no neurologic deficits. The absence of neurologic deficits in the class I-deficient mice was associated with preserved sodium channel densities in demyelinated lesions, a relative preservation of axons, and extensive spontaneous remyelination. In this study, we investigated whether TMEV-infected class II-deficient mice, which have an identical genetic background (C57BL/6 x 129) as the class I-deficient mice, have preserved axons and spontaneous myelin repair following chronic TMEV-infection. Both class I- and class II-deficient mice showed similar extents of demyelination of the spinal cord white matter 4 months after TMEV infection. However, the class I-deficient mice demonstrated remyelination by oligodendrocytes, whereas class II-deficient mice showed minimal if any myelin repair. Demyelinated lesions, characterized by inflammatory infiltrates in both mutants, revealed disruption of axons in class II- but not class I-deficient mice. Further characterization revealed that even though class II-deficient mice lacked TMEV-specific IgG, they had virus-specific IgM, which, however, did not neutralize TMEV in vitro. In addition, class II-deficient mice developed TMEV-specific cytotoxic T-lymphocytes in the CNS during the acute (7 days) disease, but these cytotoxic lymphocytes were not present in the chronic stage of disease, despite a high titer of infectious virus throughout the disease. We envision that the presence of demyelination, high virus titer, absence of remyelination, and axonal disruption in chronically infected class II-deficient mice contributes to the development of paralytic disease.
Figures





Similar articles
-
Spontaneous remyelination following extensive demyelination is associated with improved neurological function in a viral model of multiple sclerosis.Brain. 2001 Jul;124(Pt 7):1403-16. doi: 10.1093/brain/124.7.1403. Brain. 2001. PMID: 11408335 Free PMC article.
-
Perforin-dependent neurologic injury in a viral model of multiple sclerosis.J Neurosci. 1998 Sep 15;18(18):7306-14. doi: 10.1523/JNEUROSCI.18-18-07306.1998. J Neurosci. 1998. PMID: 9736651 Free PMC article.
-
Lymphocytes from mice chronically infected with Theiler's murine encephalomyelitis virus produce demyelination of organotypic cultures after stimulation with the major encephalitogenic epitope of myelin proteolipid protein. Epitope spreading in TMEV infection has functional activity.J Neuroimmunol. 2000 Apr 3;104(1):79-84. doi: 10.1016/s0165-5728(99)00230-1. J Neuroimmunol. 2000. PMID: 10683517
-
Immune promotion of central nervous system remyelination.Prog Brain Res. 1994;103:343-55. doi: 10.1016/s0079-6123(08)61148-6. Prog Brain Res. 1994. PMID: 7886217 Review.
-
[Theiler's virus encephalomyelitis infection as a model for multiple sclerosis: cytokines and pathogenic mechanisms].Rev Neurol. 2002 Nov 16-30;35(10):973-8. Rev Neurol. 2002. PMID: 12436402 Review. Spanish.
Cited by
-
Remyelination-promoting human IgMs: developing a therapeutic reagent for demyelinating disease.Curr Top Microbiol Immunol. 2008;318:213-39. doi: 10.1007/978-3-540-73677-6_9. Curr Top Microbiol Immunol. 2008. PMID: 18219820 Free PMC article. Review.
-
Human class I major histocompatibility complex alleles determine central nervous system injury versus repair.J Neuroinflammation. 2016 Nov 17;13(1):293. doi: 10.1186/s12974-016-0759-4. J Neuroinflammation. 2016. PMID: 27855706 Free PMC article.
-
Functional genomic analysis of remyelination reveals importance of inflammation in oligodendrocyte regeneration.J Neurosci. 2003 Oct 29;23(30):9824-32. doi: 10.1523/JNEUROSCI.23-30-09824.2003. J Neurosci. 2003. PMID: 14586011 Free PMC article.
-
Spontaneous remyelination following extensive demyelination is associated with improved neurological function in a viral model of multiple sclerosis.Brain. 2001 Jul;124(Pt 7):1403-16. doi: 10.1093/brain/124.7.1403. Brain. 2001. PMID: 11408335 Free PMC article.
-
Viral models of multiple sclerosis: neurodegeneration and demyelination in mice infected with Theiler's virus.Prog Neurobiol. 2013 Feb-Mar;101-102:46-64. doi: 10.1016/j.pneurobio.2012.11.003. Epub 2012 Nov 29. Prog Neurobiol. 2013. PMID: 23201558 Free PMC article.
References
-
- Ghatak NR, Leshner RT, Price AC, Felton WL. Remyelination in the human central nervous system. J Neuropath Exp Neurol. 1989;48:507–18. - PubMed
-
- Prineas JW, Barnard RO, Kwon EE, Sharer LR, Cho ES. Multiple sclerosis: Remyelination of nascent lesion. Ann Neurol. 1993;33:137–51. - PubMed
-
- Prineas JW, Kwon EE, Goldenberg PZ, Kyas AA, Quarles RH, Benjamins JA, Sprinkle TJ. Multiple sclerosis: Oligodendrocyte proliferation and differentiation in fresh lesions. Lab Invest. 1989;61:489–503. - PubMed
-
- Raine CS, Scheinberg L, Waltz JM. Multiple sclerosis: Oligodendrocytes survival and proliferation in an active established lesion. Lab Invest. 1981;45:534–46. - PubMed
-
- Raine CS, Wu E. Multiple sclerosis: Remyelination in acute lesions. J Neuropath Exp Neurol. 1993;52:199–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

