The nucleoporin nup153 plays a critical role in multiple types of nuclear export
- PMID: 10069809
- PMCID: PMC25193
- DOI: 10.1091/mbc.10.3.649
The nucleoporin nup153 plays a critical role in multiple types of nuclear export
Abstract
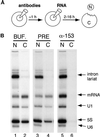
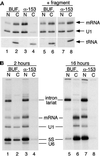
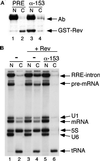
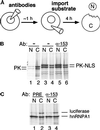
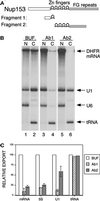
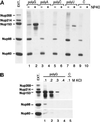
The fundamental process of nucleocytoplasmic transport takes place through the nuclear pore. Peripheral pore structures are presumably poised to interact with transport receptors and their cargo as these receptor complexes first encounter the pore. One such peripheral structure likely to play an important role in nuclear export is the basket structure located on the nuclear side of the pore. At present, Nup153 is the only nucleoporin known to localize to the surface of this basket, suggesting that Nup153 is potentially one of the first pore components an RNA or protein encounters during export. In this study, anti-Nup153 antibodies were used to probe the role of Nup153 in nuclear export in Xenopus oocytes. We found that Nup153 antibodies block three major classes of RNA export, that of snRNA, mRNA, and 5S rRNA. Nup153 antibodies also block the NES protein export pathway, specifically the export of the HIV Rev protein, as well as Rev-dependent RNA export. Not all export was blocked; Nup153 antibodies did not impede the export of tRNA or the recycling of importin beta to the cytoplasm. The specific antibodies used here also did not affect nuclear import, whether mediated by importin alpha/beta or by transportin. Overall, the results indicate that Nup153 is crucial to multiple classes of RNA and protein export, being involved at a vital juncture point in their export pathways. This juncture point appears to be one that is bypassed by tRNA during its export. We asked whether a physical interaction between RNA and Nup153 could be observed, using homoribopolymers as sequence-independent probes for interaction. Nup153, unlike four other nucleoporins including Nup98, associated strongly with poly(G) and significantly with poly(U). Thus, Nup153 is unique among the nucleoporins tested in its ability to interact with RNA and must do so either directly or indirectly through an adaptor protein. These results suggest a unique mechanistic role for Nup153 in the export of multiple cargos.
Figures
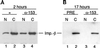
References
-
- Adam SA, Gerace L. Cytosolic proteins that specifically bind nuclear location signals are receptors for nuclear import. Cell. 1991;66:837–847. - PubMed
-
- Aitchison JD, Blobel G, Rout MP. Kap104p: a karyopherin involved in the nuclear transport of mRNA binding proteins. Science. 1996;274:624–627. - PubMed
-
- Arts GJ, Fornerod M, Mattaj IW. Identification of a nuclear export receptor for tRNA. Curr Biol. 1998;8:305–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

