Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation
- PMID: 10069811
- PMCID: PMC25195
- DOI: 10.1091/mbc.10.3.677
Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation
Abstract
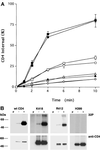
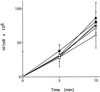
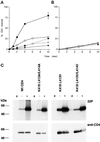
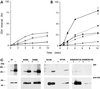
Cluster of differentiation antigen 4 (CD4), the T lymphocyte antigen receptor component and human immunodeficiency virus coreceptor, is down-modulated when cells are activated by antigen or phorbol esters. During down-modulation CD4 dissociates from p56(lck), undergoes endocytosis through clathrin-coated pits, and is then sorted in early endosomes to late endocytic organelles where it is degraded. Previous studies have suggested that phosphorylation and a dileucine sequence are required for down-modulation. Using transfected HeLa cells, in which CD4 endocytosis can be studied in the absence of p56(lck), we show that the dileucine sequence in the cytoplasmic domain is essential for clathrin-mediated CD4 endocytosis. However, this sequence is only functional as an endocytosis signal when neighboring serine residues are phosphorylated. Phosphoserine is required for rapid endocytosis because CD4 molecules in which the cytoplasmic domain serine residues are substituted with glutamic acid residues are not internalized efficiently. Using surface plasmon resonance, we show that CD4 peptides containing the dileucine sequence bind weakly to clathrin adaptor protein complexes 2 and 1. The affinity of this interaction is increased 350- to 700-fold when the peptides also contain phosphoserine residues.
Figures



References
-
- Bar-Zvi D, Mosley ST, Branton D. In vivo phosphorylation of clathrin-coated vesicle proteins from rat reticulocytes. J Biol Chem. 1998;263:4408–4415. - PubMed
-
- Bremnes T, Lauvrak V, Lindqvist B, Bakke O. A region from the medium chain adaptor subunit (μ) recognizes leucine- and tyrosine-based sorting signals. J Biol Chem. 1998;273:8638–8645. - PubMed
-
- Bremnes B, Madsen T, Geddedahl M, Bakke O. An LI and ML motif in the cytoplasmic tail of the MHC- associated invariant chain mediate rapid internalization. J Cell Sci. 1994;107:2021–2032. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

