The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains
- PMID: 10069814
- PMCID: PMC25198
- DOI: 10.1091/mbc.10.3.727
The development of cell processes induced by tau protein requires phosphorylation of serine 262 and 356 in the repeat domain and is inhibited by phosphorylation in the proline-rich domains
Abstract
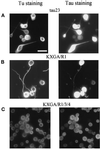
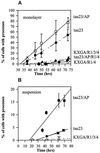
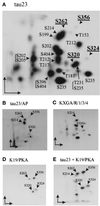
The differentiation of neurons and the outgrowth of neurites depends on microtubule-associated proteins such as tau protein. To study this process, we have used the model of Sf9 cells, which allows efficient transfection with microtubule-associated proteins (via baculovirus vectors) and observation of the resulting neurite-like extensions. We compared the phosphorylation of tau23 (the embryonic form of human tau) with mutants in which critical phosphorylation sites were deleted by mutating Ser or Thr residues into Ala. One can broadly distinguish two types of sites, the KXGS motifs in the repeats (which regulate the affinity of tau to microtubules) and the SP or TP motifs in the domains flanking the repeats (which contain epitopes for antibodies diagnostic of Alzheimer's disease). Here we report that both types of sites can be phosphorylated by endogenous kinases of Sf9 cells, and that the phosphorylation pattern of the transfected tau is very similar to that of neurons, showing that Sf9 cells can be regarded as an approximate model for the neuronal balance between kinases and phosphatases. We show that mutations in the repeat domain and in the flanking domains have opposite effects. Mutations of KXGS motifs in the repeats (Ser262, 324, and 356) strongly inhibit the outgrowth of cell extensions induced by tau, even though this type of phosphorylation accounts for only a minor fraction of the total phosphate. This argues that the temporary detachment of tau from microtubules (by phosphorylation at KXGS motifs) is a necessary condition for establishing cell polarity at a critical point in space or time. Conversely, the phosphorylation at SP or TP motifs represents the majority of phosphate (>80%); mutations in these motifs cause an increase in cell extensions, indicating that this type of phosphorylation retards the differentiation of the cells.
Figures




Similar articles
-
Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments.Biochemistry. 1999 Mar 23;38(12):3549-58. doi: 10.1021/bi981874p. Biochemistry. 1999. PMID: 10090741
-
Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity.Mol Biol Cell. 2002 Nov;13(11):4013-28. doi: 10.1091/mbc.02-03-0046. Mol Biol Cell. 2002. PMID: 12429843 Free PMC article.
-
Domains of tau protein, differential phosphorylation, and dynamic instability of microtubules.Mol Biol Cell. 1995 Dec;6(12):1887-902. doi: 10.1091/mbc.6.12.1887. Mol Biol Cell. 1995. PMID: 8590813 Free PMC article.
-
Tau domains, phosphorylation, and interactions with microtubules.Neurobiol Aging. 1995 May-Jun;16(3):355-62; discussion 362-3. doi: 10.1016/0197-4580(95)00025-a. Neurobiol Aging. 1995. PMID: 7566345 Review.
-
Microtubule-associated protein tau, paired helical filaments, and phosphorylation.Ann N Y Acad Sci. 1993 Sep 24;695:209-16. doi: 10.1111/j.1749-6632.1993.tb23054.x. Ann N Y Acad Sci. 1993. PMID: 7694533 Review.
Cited by
-
Identification of oligomers at early stages of tau aggregation in Alzheimer's disease.FASEB J. 2012 May;26(5):1946-59. doi: 10.1096/fj.11-199851. Epub 2012 Jan 17. FASEB J. 2012. PMID: 22253473 Free PMC article.
-
Axonal transport, tau protein, and neurodegeneration in Alzheimer's disease.Neuromolecular Med. 2002;2(2):151-65. doi: 10.1385/NMM:2:2:151. Neuromolecular Med. 2002. PMID: 12428809 Review.
-
The Implication of Glial Metabotropic Glutamate Receptors in Alzheimer's Disease.Curr Neuropharmacol. 2023;21(2):164-182. doi: 10.2174/1570159X20666211223140303. Curr Neuropharmacol. 2023. PMID: 34951388 Free PMC article. Review.
-
Conformational signatures induced by ubiquitin modification in the amyloid-forming tau repeat domain.Proc Natl Acad Sci U S A. 2025 Apr 15;122(15):e2425831122. doi: 10.1073/pnas.2425831122. Epub 2025 Apr 8. Proc Natl Acad Sci U S A. 2025. PMID: 40198698
-
A pathological phosphorylation pattern enhances tau cooperativity on microtubules and facilitates tau filament assembly.Res Sq [Preprint]. 2025 Apr 10:rs.3.rs-6247226. doi: 10.21203/rs.3.rs-6247226/v1. Res Sq. 2025. PMID: 40297677 Free PMC article. Preprint.
References
-
- Baas P, Pienkowski T, Cimbalnik K, Toyama K, Bakalis S, Ahmad F, Kosik K. Tau confers drug stability but not cold stability to microtubules in living cells. J Cell Sci. 1994;107:135–143. - PubMed
-
- Belmont LD, Hyman AA, Sawin KE, Mitchison TJ. Real-time visualization of cell-cycle dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990;62:579–589. - PubMed
-
- Biernat J, Gustke N, Drewes G, Mandelkow E-M, Mandelkow E. Phosphorylation of serine 262 strongly reduces the binding of tau protein to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

