Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation
- PMID: 10069818
- PMCID: PMC25202
- DOI: 10.1091/mbc.10.3.785
Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation
Abstract
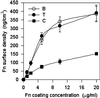
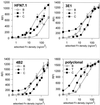
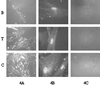
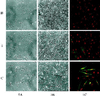
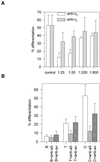
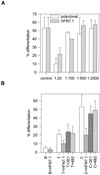
Integrin-mediated cell adhesion to extracellular matrices provides signals essential for cell cycle progression and differentiation. We demonstrate that substrate-dependent changes in the conformation of adsorbed fibronectin (Fn) modulated integrin binding and controlled switching between proliferation and differentiation. Adsorption of Fn onto bacterial polystyrene (B), tissue culture polystyrene (T), and collagen (C) resulted in differences in Fn conformation as indicated by antibody binding. Using a biochemical method to quantify bound integrins in cultured cells, we found that differences in Fn conformation altered the quantity of bound alpha5 and beta1 integrin subunits but not alphav or beta3. C2C12 myoblasts grown on these Fn-coated substrates proliferated to different levels (B > T > C). Immunostaining for muscle-specific myosin revealed minimal differentiation on B, significant levels on T, and extensive differentiation on C. Differentiation required binding to the RGD cell binding site in Fn and was blocked by antibodies specific for this site. Switching between proliferation and differentiation was controlled by the levels of alpha5beta1 integrin bound to Fn, and differentiation was inhibited by anti-alpha5, but not anti-alphav, antibodies, suggesting distinct integrin-mediated signaling pathways. Control of cell proliferation and differentiation through conformational changes in extracellular matrix proteins represents a versatile mechanism to elicit specific cellular responses for biological and biotechnological applications.
Figures

Similar articles
-
Enhanced expression of the osteoblastic phenotype on substrates that modulate fibronectin conformation and integrin receptor binding.Biomaterials. 2002 Jun;23(12):2527-34. doi: 10.1016/s0142-9612(01)00387-8. Biomaterials. 2002. PMID: 12033600
-
Modulation of calcium current in arteriolar smooth muscle by alphav beta3 and alpha5 beta1 integrin ligands.J Cell Biol. 1998 Oct 5;143(1):241-52. doi: 10.1083/jcb.143.1.241. J Cell Biol. 1998. PMID: 9763435 Free PMC article.
-
Surface chemistry modulates fibronectin conformation and directs integrin binding and specificity to control cell adhesion.J Biomed Mater Res A. 2003 Aug 1;66(2):247-59. doi: 10.1002/jbm.a.10537. J Biomed Mater Res A. 2003. PMID: 12888994
-
Fibronectin polymerization stimulates cell growth by RGD-dependent and -independent mechanisms.J Cell Sci. 2000 Dec;113 Pt 23:4287-99. doi: 10.1242/jcs.113.23.4287. J Cell Sci. 2000. PMID: 11069773
-
Roles of integrins in fibronectin matrix assembly.Histol Histopathol. 1997 Jan;12(1):233-40. Histol Histopathol. 1997. PMID: 9046058 Review.
Cited by
-
Development of a 3-D Physical Dynamics Monitoring System Using OCM with DVC for Quantification of Sprouting Endothelial Cells Interacting with a Collagen Matrix.Materials (Basel). 2020 Jun 12;13(12):2693. doi: 10.3390/ma13122693. Materials (Basel). 2020. PMID: 32545667 Free PMC article.
-
The landscape of photoaging: From bench to bedside in a bibliometric analysis.Front Public Health. 2022 Oct 20;10:972766. doi: 10.3389/fpubh.2022.972766. eCollection 2022. Front Public Health. 2022. PMID: 36339199 Free PMC article.
-
Nanoscale features of fibronectin fibrillogenesis depend on protein-substrate interaction and cytoskeleton structure.Biophys J. 2005 Jan;88(1):527-34. doi: 10.1529/biophysj.104.048074. Epub 2004 Nov 8. Biophys J. 2005. PMID: 15533920 Free PMC article.
-
Identification of the proliferation/differentiation switch in the cellular network of multicellular organisms.PLoS Comput Biol. 2006 Nov 24;2(11):e145. doi: 10.1371/journal.pcbi.0020145. PLoS Comput Biol. 2006. PMID: 17166053 Free PMC article.
-
Substrate-dependent morphology of supramolecular assemblies: fibrillin and type-VI collagen microfibrils.Biophys J. 2004 May;86(5):3211-22. doi: 10.1016/S0006-3495(04)74369-6. Biophys J. 2004. PMID: 15111434 Free PMC article.
References
-
- Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes α5β1 integrin loss from the cell surface. Cell. 1990;63:425–435. - PubMed
-
- Aota S, Nomizu M, Yamada KM. The short amino acid sequence ProHis-Ser-Arg-Asn in human fibronectin enhances cell-adhesive function. J Biol Chem. 1994;269:24756–24761. - PubMed
-
- Bischoff R, Holtzer H. The effect of mitotic inhibitors on myogenesis in vitro. J Cell Biol. 1968;36:111–127. - PubMed
-
- Blaschuk KL, Holland PC. The regulation of α5β1 integrin expression in human muscle cells. Dev Biol. 1994;164:475–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

