NADH-glutamate synthase in alfalfa root nodules. Genetic regulation and cellular expression
- PMID: 10069821
- PMCID: PMC32097
- DOI: 10.1104/pp.119.3.817
NADH-glutamate synthase in alfalfa root nodules. Genetic regulation and cellular expression
Abstract
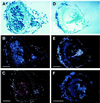
NADH-dependent glutamate synthase (NADH-GOGAT; EC 1.4.1.14) is a key enzyme in primary nitrogen assimilation in alfalfa (Medicago sativa L.) root nodules. Here we report that in alfalfa, a single gene, probably with multiple alleles, encodes for NADH-GOGAT. In situ hybridizations were performed to assess the location of NADH-GOGAT transcript in alfalfa root nodules. In wild-type cv Saranac nodules the NADH-GOGAT gene is predominantly expressed in infected cells. Nodules devoid of bacteroids (empty) induced by Sinorhizobium meliloti 7154 had no NADH-GOGAT transcript detectable by in situ hybridization, suggesting that the presence of the bacteroid may be important for NADH-GOGAT expression. The pattern of expression of NADH-GOGAT shifted during root nodule development. Until d 9 after planting, all infected cells appeared to express NADH-GOGAT. By d 19, a gradient of expression from high in the early symbiotic zone to low in the late symbiotic zone was observed. In 33-d-old nodules expression was seen in only a few cell layers in the early symbiotic zone. This pattern of expression was also observed for the nifH transcript but not for leghemoglobin. The promoter of NADH-GOGAT was evaluated in transgenic alfalfa plants carrying chimeric beta-glucuronidase promoter fusions. The results suggest that there are at least four regulatory elements. The region responsible for expression in the infected cell zone contains an 88-bp direct repeat.
Figures






References
-
- Austin S, Bingham ET, Mathews DE, Shahan MN, Will J, Burgess RR. Production and field performance of transgenic alfalfa (Medicago sativa L.) expressing alpha-amylase and manganese-dependent lignin peroxidase. Euphytica. 1995;85:381–383.
-
- Avila C, Marquez AJ, Pajuelo P, Cannell ME, Wallsgrove RM, Forde BG. Cloning and sequence analysis of a cDNA for barley ferredoxin-dependent glutamate synthase and molecular analysis of photorespiratory mutants deficient in the enzyme. Planta. 1993;189:475–483. - PubMed
-
- Becker TW, Perrot-Rechenmann C, Suzuki A, Hirel B. Subcellular and immunocytochemical localization of the enzymes involved in ammonia assimilation in mesophyll and bundle-sheath cells of maize leaves. Planta. 1993;191:129–136.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

