NADH-Glutamate synthase in alfalfa root nodules. Immunocytochemical localization
- PMID: 10069822
- PMCID: PMC32098
- DOI: 10.1104/pp.119.3.829
NADH-Glutamate synthase in alfalfa root nodules. Immunocytochemical localization
Abstract
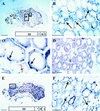
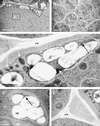
In root nodules of alfalfa (Medicago sativa L.), N2 is reduced to NH4+ in the bacteroid by the nitrogenase enzyme and then released into the plant cytosol. The NH4+ is then assimilated by the combined action of glutamine synthetase (EC 6.3.1.2) and NADH-dependent Glu synthase (NADH-GOGAT; EC 1.4.1.14) into glutamine and Glu. The alfalfa nodule NADH-GOGAT protein has a 101-amino acid presequence, but the subcellular location of the protein is unknown. Using immunocytochemical localization, we determined first that the NADH-GOGAT protein is found throughout the infected cell region of both 19- and 33-d-old nodules. Second, in alfalfa root nodules NADH-GOGAT is localized predominantly to the amyloplast of infected cells. This finding, together with earlier localization and fractionation studies, indicates that in alfalfa the infected cells are the main location for the initial assimilation of fixed N2.
Figures


Similar articles
-
Decreased NADH glutamate synthase activity in nodules and flowers of alfalfa (Medicago sativa L.) transformed with an antisense glutamate synthase transgene.J Exp Bot. 2000 Jan;51(342):29-39. J Exp Bot. 2000. PMID: 10938793
-
NADH-glutamate synthase in alfalfa root nodules. Genetic regulation and cellular expression.Plant Physiol. 1999 Mar;119(3):817-28. doi: 10.1104/pp.119.3.817. Plant Physiol. 1999. PMID: 10069821 Free PMC article.
-
Alfalfa NADH-dependent glutamate synthase: structure of the gene and importance in symbiotic N2 fixation.Plant J. 1995 Sep;8(3):345-58. doi: 10.1046/j.1365-313x.1995.08030345.x. Plant J. 1995. PMID: 7550373
-
Cellular compartmentation of ammonium assimilation in rice and barley.J Exp Bot. 2001 Apr;52(356):591-604. J Exp Bot. 2001. PMID: 11373307 Review.
-
Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice.J Exp Bot. 2002 Apr;53(370):917-25. doi: 10.1093/jexbot/53.370.917. J Exp Bot. 2002. PMID: 11912234 Review.
Cited by
-
Nutrient sharing between symbionts.Plant Physiol. 2007 Jun;144(2):604-14. doi: 10.1104/pp.107.097741. Plant Physiol. 2007. PMID: 17556524 Free PMC article. Review. No abstract available.
-
Cellular expression and regulation of the Medicago truncatula cytosolic glutamine synthetase genes in root nodules.Plant Mol Biol. 2000 Mar;42(5):741-56. doi: 10.1023/a:1006304003770. Plant Mol Biol. 2000. PMID: 10809446
-
Okadaic Acid Mimics Nitrogen-Stimulated Transcription of the NADH-Glutamate Synthase Gene in Rice Cell Cultures.Plant Physiol. 1999 Nov;121(3):805-812. doi: 10.1104/pp.121.3.805. Plant Physiol. 1999. PMID: 10557228 Free PMC article.
-
Microcystin-tolerant Rhizobium protects plants and improves nitrogen assimilation in Vicia faba irrigated with microcystin-containing waters.Environ Sci Pollut Res Int. 2016 May;23(10):10037-49. doi: 10.1007/s11356-016-6223-2. Epub 2016 Feb 11. Environ Sci Pollut Res Int. 2016. PMID: 26865488
-
Glutamate synthase: structural, mechanistic and regulatory properties, and role in the amino acid metabolism.Photosynth Res. 2005;83(2):191-217. doi: 10.1007/s11120-004-3478-0. Photosynth Res. 2005. PMID: 16143852 Review.
References
-
- Becker TW, Rechenmann CP, Suzuki A, Hirel B. Subcellular and immunocytochemical localization of the enzymes involved in ammonia assimilation in mesophyll and bundle-sheath cells of maize leaves. Planta. 1993;191:129–136.
-
- Bernhard WR, Matile P. Differential expression of glutamine synthetase genes during the senescence of Arabidopsis thaliana rosette leaves. Plant Sci. 1994;98:7–14.
-
- Bisseling T, Van Straten J, Houwaard F. Turnover of nitrogenase and leghemoglobin in root nodules of Pisum sativum. Biochim Biophys Acta. 1980;610:360–370. - PubMed
-
- Boland MJ, Benny AG. Enzymes of nitrogen metabolism in legume nodules: purification and properties of NADH-dependent Glu synthase from lupine nodules. Eur J Biochem. 1977;79:355–362. - PubMed
LinkOut - more resources
Full Text Sources

