A multisubunit acetyl coenzyme A carboxylase from soybean
- PMID: 10069834
- PMCID: PMC32110
- DOI: 10.1104/pp.119.3.961
A multisubunit acetyl coenzyme A carboxylase from soybean
Abstract
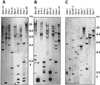
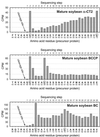
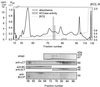
A multisubunit form of acetyl coenzyme A (CoA) carboxylase (ACCase) from soybean (Glycine max) was characterized. The enzyme catalyzes the formation of malonyl CoA from acetyl CoA, a rate-limiting step in fatty acid biosynthesis. The four known components that constitute plastid ACCase are biotin carboxylase (BC), biotin carboxyl carrier protein (BCCP), and the alpha- and beta-subunits of carboxyltransferase (alpha- and beta-CT). At least three different cDNAs were isolated from germinating soybean seeds that encode BC, two that encode BCCP, and four that encode alpha-CT. Whereas BC, BCCP, and alpha-CT are products of nuclear genes, the DNA that encodes soybean beta-CT is located in chloroplasts. Translation products from cDNAs for BC, BCCP, and alpha-CT were imported into isolated pea (Pisum sativum) chloroplasts and became integrated into ACCase. Edman microsequence analysis of the subunits after import permitted the identification of the amino-terminal sequence of the mature protein after removal of the transit sequences. Antibodies specific for each of the chloroplast ACCase subunits were generated against products from the cDNAs expressed in bacteria. The antibodies permitted components of ACCase to be followed during fractionation of the chloroplast stroma. Even in the presence of 0.5 M KCl, a complex that contained BC plus BCCP emerged from Sephacryl 400 with an apparent molecular mass greater than about 800 kD. A second complex, which contained alpha- and beta-CT, was also recovered from the column, and it had an apparent molecular mass of greater than about 600 kD. By mixing the two complexes together at appropriate ratios, ACCase enzymatic activity was restored. Even higher ACCase activities were recovered by mixing complexes from pea and soybean. The results demonstrate that the active form of ACCase can be reassembled and that it could form a high-molecular-mass complex.
Figures









Similar articles
-
The multisubunit acetyl-CoA carboxylase is strongly associated with the chloroplast envelope through non-ionic interactions to the carboxyltransferase subunits.Arch Biochem Biophys. 2002 Apr 15;400(2):245-57. doi: 10.1016/S0003-9861(02)00025-5. Arch Biochem Biophys. 2002. PMID: 12054435
-
Biotin carboxyl carrier protein and carboxyltransferase subunits of the multi-subunit form of acetyl-CoA carboxylase from Brassica napus: cloning and analysis of expression during oilseed rape embryogenesis.Biochem J. 1996 Apr 1;315 ( Pt 1)(Pt 1):103-12. doi: 10.1042/bj3150103. Biochem J. 1996. PMID: 8670092 Free PMC article.
-
Chloroflexus aurantiacus acetyl-CoA carboxylase evolves fused biotin carboxylase and biotin carboxyl carrier protein to complete carboxylation activity.mBio. 2024 May 8;15(5):e0341423. doi: 10.1128/mbio.03414-23. Epub 2024 Apr 4. mBio. 2024. PMID: 38572988 Free PMC article.
-
Comprehensive guide to acetyl-carboxylases in algae.Crit Rev Biotechnol. 2013 Mar;33(1):49-65. doi: 10.3109/07388551.2012.668671. Epub 2012 Apr 23. Crit Rev Biotechnol. 2013. PMID: 22524446 Review.
-
Regulation and structure of the heteromeric acetyl-CoA carboxylase.Biochim Biophys Acta. 2016 Sep;1861(9 Pt B):1207-1213. doi: 10.1016/j.bbalip.2016.04.004. Epub 2016 Apr 16. Biochim Biophys Acta. 2016. PMID: 27091637 Review.
Cited by
-
Coordinate regulation of the nuclear and plastidic genes coding for the subunits of the heteromeric acetyl-coenzyme A carboxylase.Plant Physiol. 2000 Apr;122(4):1057-71. doi: 10.1104/pp.122.4.1057. Plant Physiol. 2000. PMID: 10759501 Free PMC article.
-
Fatty acid biosynthesis in mitochondria of grasses: malonyl-coenzyme A is generated by a mitochondrial-localized acetyl-coenzyme A carboxylase.Plant Physiol. 2003 Oct;133(2):875-84. doi: 10.1104/pp.103.027375. Epub 2003 Sep 11. Plant Physiol. 2003. PMID: 12972648 Free PMC article.
-
MYB89 Transcription Factor Represses Seed Oil Accumulation.Plant Physiol. 2017 Feb;173(2):1211-1225. doi: 10.1104/pp.16.01634. Epub 2016 Dec 8. Plant Physiol. 2017. PMID: 27932421 Free PMC article.
-
Protein interactomes for plant lipid biosynthesis and their biotechnological applications.Plant Biotechnol J. 2023 Sep;21(9):1734-1744. doi: 10.1111/pbi.14027. Epub 2023 Mar 9. Plant Biotechnol J. 2023. PMID: 36762506 Free PMC article. Review.
-
Draft genome sequence and detailed characterization of biofuel production by oleaginous microalga Scenedesmus quadricauda LWG002611.Biotechnol Biofuels. 2018 Nov 9;11:308. doi: 10.1186/s13068-018-1308-4. eCollection 2018. Biotechnol Biofuels. 2018. PMID: 30455737 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

