Impacts of aluminum on the cytoskeleton of the maize root apex. short-term effects on the distal part of the transition zone
- PMID: 10069846
- PMCID: PMC32089
- DOI: 10.1104/pp.119.3.1073
Impacts of aluminum on the cytoskeleton of the maize root apex. short-term effects on the distal part of the transition zone
Abstract
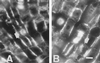
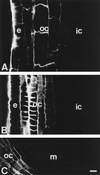
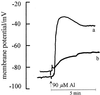
Using monoclonal tubulin and actin antibodies, Al-mediated alterations to microtubules (MTs) and actin microfilaments (MFs) were shown to be most prominent in cells of the distal part of the transition zone (DTZ) of an Al-sensitive maize (Zea mays L.) cultivar. An early response to Al (1 h, 90 μM) was the depletion of MTs in cells of the DTZ, specifically in the outermost cortical cell file. However, no prominent changes to the MT cytoskeleton were found in elongating cells treated with Al for 1 h in spite of severe inhibition of root elongation. Al-induced early alterations to actin MFs were less dramatic and consisted of increased actin fluorescence of partially disintegrated MF arrays in cells of the DTZ. These tissue- and development-specific alterations to the cytoskeleton were preceded by and/or coincided with Al-induced depolarization of the plasma membrane and with callose formation, particularly in the outer cortex cells of the DTZ. Longer Al supplies (>6 h) led to progressive enhancements of lesions to the MT cytoskeleton in the epidermis and two to three outer cortex cell files. Our data show that the cytoskeleton in the cells of the DTZ is especially sensitive to Al, consistent with the recently proposed specific Al sensitivity of this unique, apical maize root zone.
Figures





References
-
- Alfano F, Russell A, Gambardella R, Duckett JG. The actin cytoskeleton of the liverwort Riccia fluitans: effects of cytochalasin B and aluminium ions on rhizoid tip growth. J Plant Physiol. 1993;142:569–574.
-
- Baluška F, Barlow PW, Hauskrecht M, Kubica Š, Parker JS, Volkmann D. Microtubule arrays in maize root cells: interplay between the cytoskeleton, nuclear organization and postmitotic cellular growth patterns. New Phytol. 1995;130:177–192.
-
- Baluška F, Barlow PW, Volkmann D. Complete disintegration of the microtubular cytoskeleton precedes auxin-mediated reconstruction in postmitotic maize root cells. Plant Cell Physiol. 1996a;37:1013–1021. - PubMed
-
- Baluška F, Brailsford RW, Hauskrecht M, Jackson MB, Barlow PW. Cellular dimorphism in the maize root cortex: involvement of microtubules, ethylene and gibberelin in the differentiation of cellular behaviour in postmitotic growth zones. Bot Acta. 1993a;106:394–403.
-
- Baluška F, Hasenstein KH. Root cytoskeleton: its role in perception of and response to gravity. Planta. 1997;203:S-69–78. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

