Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants
- PMID: 10069850
- PMCID: PMC32093
- DOI: 10.1104/pp.119.3.1107
Nicotianamine chelates both FeIII and FeII. Implications for metal transport in plants
Abstract
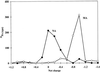
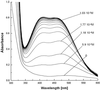
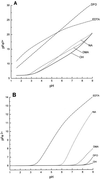
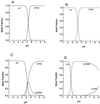
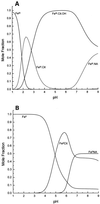
Nicotianamine (NA) occurs in all plants and chelates metal cations, including FeII, but reportedly not FeIII. However, a comparison of the FeII and ZnII affinity constants of NA and various FeIII-chelating aminocarboxylates suggested that NA should chelate FeIII. High-voltage electrophoresis of the FeNA complex formed in the presence of FeIII showed that the complex had a net charge of 0, consistent with the hexadentate chelation of FeIII. Measurement of the affinity constant for FeIII yielded a value of 10(20.6), which is greater than that for the association of NA with FeII (10(12.8)). However, capillary electrophoresis showed that in the presence of FeII and FeIII, NA preferentially chelates FeII, indicating that the FeIINA complex is kinetically stable under aerobic conditions. Furthermore, Fe complexes of NA are relatively poor Fenton reagents, as measured by their ability to mediate H2O2-dependent oxidation of deoxyribose. This suggests that NA will have an important role in scavenging Fe and protecting the cell from oxidative damage. The pH dependence of metal ion chelation by NA and a typical phytosiderophore, 2'-deoxymugineic acid, indicated that although both have the ability to chelate Fe, when both are present, 2'-deoxymugineic acid dominates the chelation process at acidic pH values, whereas NA dominates at alkaline pH values. The consequences for the role of NA in the long-distance transport of metals in the xylem and phloem are discussed.
Figures





References
-
- Anderegg G, L'Eplattenier F, Schwarzenbach G. Hydroxamatkomplexe III. Eisen(III)-austansch zwischen sideraminen und komplexen. Discussion der bildungskonstanten der hydroxamatkomplexe. Helv Chim Acta. 1963;46:1409–1422.
-
- Anderegg G, Ripperger H (1989) Correlation between metal complex formation and biological activity of nicotianamine analogues. J Chem Soc Chem Commun 647–650
-
- Beneš I, Schreiber K, Ripperger H, Kircheiss A. Metal complex formation by nicotianamine, a possible phytosiderophore. Experientia. 1983;39:261–262.
-
- Gran G. Determination of equivalence point in potentiometric titrations: part II. Analyst. 1952;77:661–671.
LinkOut - more resources
Full Text Sources
Other Literature Sources

