Nuclear import of the Drosophila Rel protein Dorsal is regulated by phosphorylation
- PMID: 10072384
- PMCID: PMC316510
- DOI: 10.1101/gad.13.5.556
Nuclear import of the Drosophila Rel protein Dorsal is regulated by phosphorylation
Abstract
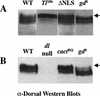
In Drosophila, dorsal-ventral polarity is determined by a maternally encoded signal transduction pathway that culminates in the graded nuclear localization of the Rel protein, Dorsal. Dorsal is retained in the cytoplasm by the IkappaB protein, Cactus. Signal-dependent phosphorylation of Cactus results in the degradation of Cactus and the nuclear targeting of Dorsal. We present an in-depth study of the functional importance of Dorsal phosphorylation. We find that Dorsal is phosphorylated by the ventral signal while associated with Cactus, and that Dorsal phosphorylation is essential for its nuclear import. In vivo phospholabeling of Dorsal is limited to serine residues in both ovaries and early embryos. A protein bearing mutations in six conserved serines abolishes Dorsal activity, is constitutively cytoplasmic, and appears to eliminate Dorsal phosphorylation, but still interacts with Cactus. Two individual serine-to-alanine mutations produce unexpected results. In a wild-type signaling background, a mutation in the highly conserved PKA site (S312) produces only a weak loss-of-function; however, it completely destabilizes the protein in a cactus mutant background. Significantly, the phosphorylation of another completely conserved serine (S317) regulates the high level of nuclear import found in ventral cells. We conclude that the formation of a wild-type Dorsal nuclear gradient requires the phosphorylation of both Cactus and Dorsal. The strong conservation of the serines suggests that phosphorylation of other Rel proteins is essential for their proper nuclear targeting.
Figures






References
-
- Anderson KV, Nüsslein-Volhard C. Genetic analysis of dorsal-ventral embryonic pattern in Drosophila. In: Molacinski GM, Bryant SV, editors. Pattern formation. New York, NY: MacMillan; 1984. pp. 269–289.
-
- Ashburner M. Drosophila: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
-
- Baeuerle PA, Baltimore D. Activation of DNA-binding activity in an apparently cytoplasmic precursor of NF-κB transcription factor. Cell. 1998;53:211–217. - PubMed
-
- Belvin MP, Jin Y, Anderson KV. Cactus protein degradation mediates Drosophila dorsal–ventral signaling. Genes & Dev. 1995;9:783–793. - PubMed
-
- Bergmann A, Stein D, Geisler R, Hagenmaier S, Schmid B, Fernandez N, Schnell B, Nüsslein-Volhard C. A gradient of cytoplasmic Cactus degradation establishes the nuclear localization gradient of the dorsal morphogen in Drosophila. Mech Dev. 1996;60:109–123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
