Binding of hnRNP H to an exonic splicing silencer is involved in the regulation of alternative splicing of the rat beta-tropomyosin gene
- PMID: 10072387
- PMCID: PMC316507
- DOI: 10.1101/gad.13.5.593
Binding of hnRNP H to an exonic splicing silencer is involved in the regulation of alternative splicing of the rat beta-tropomyosin gene
Abstract
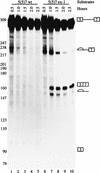
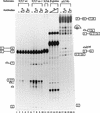
In the rat beta-tropomyosin (beta-TM) gene, exons 6 and 7 are spliced alternatively in a mutually exclusive manner. Exon 6 is included in mRNA encoding nonmuscle TM-1, whereas exon 7 is used in mRNA encoding skeletal muscle beta-TM. Previously, we demonstrated that a six nucleotide mutation at the 5' end of exon 7, designated as ex-1, activated exon 7 splicing in nonmuscle cells. In this study, we show that the activating effect of this mutation is not the result of creating an exonic splicing enhancer (ESE) or disrupting a putative secondary structure. The sequence in exon 7 acts as a bona fide exonic splicing silencer (ESS), which is bound specifically by a trans-acting factor. Isolation and peptide sequencing reveal that this factor is hnRNP H, a member of the heterogeneous nuclear ribonucleoprotein (hnRNP) family. Binding of hnRNP H correlates with the ESS activity. Furthermore, addition of antibodies that specifically recognizes hnRNP H to the splicing reactions or partial depletion of hnRNP H from nuclear extract activates exon 7 splicing in vitro and this effect can be reversed by addition of purified recombinant hnRNP H. These results indicate that hnRNP H participates in exclusion of exon 7 in nonmuscle cells. The involvement of hnRNP H in the activity of an ESS may represent a prototype for the regulation of tissue- and developmental-specific alternative splicing.
Figures











Similar articles
-
hnRNP A1 and the SR proteins ASF/SF2 and SC35 have antagonistic functions in splicing of beta-tropomyosin exon 6B.J Biol Chem. 2004 Sep 10;279(37):38249-59. doi: 10.1074/jbc.M405377200. Epub 2004 Jun 18. J Biol Chem. 2004. PMID: 15208309
-
Heterogeneous nuclear ribonucleoprotein (hnRNP) K is a component of an intronic splicing enhancer complex that activates the splicing of the alternative exon 6A from chicken beta-tropomyosin pre-mRNA.J Biol Chem. 2002 May 10;277(19):16614-23. doi: 10.1074/jbc.M201083200. Epub 2002 Feb 26. J Biol Chem. 2002. PMID: 11867641
-
Modulation of exon skipping and inclusion by heterogeneous nuclear ribonucleoprotein A1 and pre-mRNA splicing factor SF2/ASF.Mol Cell Biol. 1993 May;13(5):2993-3001. doi: 10.1128/mcb.13.5.2993-3001.1993. Mol Cell Biol. 1993. PMID: 8474457 Free PMC article.
-
Tissue-specific splicing of two mutually exclusive exons of the chicken beta-tropomyosin pre-mRNA: positive and negative regulations.Biochimie. 1996;78(6):457-65. doi: 10.1016/0300-9084(96)84752-3. Biochimie. 1996. PMID: 8915535 Review.
-
Role of viral splicing elements and cellular RNA binding proteins in regulation of HIV-1 alternative RNA splicing.Curr HIV Res. 2006 Jan;4(1):43-55. doi: 10.2174/157016206775197655. Curr HIV Res. 2006. PMID: 16454710 Review.
Cited by
-
Muscle-specific splicing factors ASD-2 and SUP-12 cooperatively switch alternative pre-mRNA processing patterns of the ADF/cofilin gene in Caenorhabditis elegans.PLoS Genet. 2012;8(10):e1002991. doi: 10.1371/journal.pgen.1002991. Epub 2012 Oct 11. PLoS Genet. 2012. PMID: 23071450 Free PMC article.
-
Comprehensive map of age-associated splicing changes across human tissues and their contributions to age-associated diseases.Sci Rep. 2018 Jul 19;8(1):10929. doi: 10.1038/s41598-018-29086-2. Sci Rep. 2018. PMID: 30026530 Free PMC article.
-
Depletion of TDP 43 overrides the need for exonic and intronic splicing enhancers in the human apoA-II gene.Nucleic Acids Res. 2005 Oct 27;33(18):6000-10. doi: 10.1093/nar/gki897. Print 2005. Nucleic Acids Res. 2005. PMID: 16254078 Free PMC article.
-
Differential alternative splicing activity of isoforms of polypyrimidine tract binding protein (PTB).RNA. 2001 Jun;7(6):819-32. doi: 10.1017/s1355838201010214. RNA. 2001. PMID: 11421360 Free PMC article.
-
Splicing dysregulation contributes to the pathogenicity of several F9 exonic point variants.Mol Genet Genomic Med. 2019 Aug;7(8):e840. doi: 10.1002/mgg3.840. Epub 2019 Jun 30. Mol Genet Genomic Med. 2019. PMID: 31257730 Free PMC article.
References
-
- Adams MD, Rudner DZ, Rio DC. Biochemistry and regulation of pre-mRNA splicing. Curr Opin Cell Biol. 1996;8:331–339. - PubMed
-
- Black D. Activation of c-src neuron-specific splicing by an unusual RNA element in vitro and in vivo. Cell. 1992;69:795–807. - PubMed
-
- Boggs RT, Gregor P, Idriss S, Belote JM, McKeown M. Regulation of sexual differentiation in D. melanogaster via alternative splicing of RNA from the transformer gene. Cell. 1987;50:739–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
