Differential vascular alpha1-adrenoceptor antagonism by tamsulosin and terazosin
- PMID: 10073742
- PMCID: PMC2014197
- DOI: 10.1046/j.1365-2125.1999.00856.x
Differential vascular alpha1-adrenoceptor antagonism by tamsulosin and terazosin
Abstract
Aims: In patients with lower urinary tract symptoms suggestive of benign prostatic obstruction the alpha1-adrenoceptor antagonist terazosin lowers blood pressure whereas only very small if any alterations were reported with the alpha1-adrenoceptor antagonist tamsulosin. Therefore, we have compared the vascular alpha1-adrenoceptor antagonism of tamsulosin and terazosin directly.
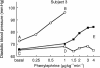
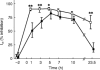
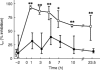
Methods: Ten healthy subjects were investigated in a randomized, single-blind, three-way cross-over design and received a single dose of 0.4 mg tamsulosin, 5 mg terazosin or placebo on 3 study days at least 1 week apart. Before and 1, 3, 5, 7, 10 and 23.5 h after drug intake, alterations of diastolic blood pressure and other haemodynamic parameters in response to a graded infusion of the alpha1-adrenoceptor agonist phenylephrine were determined non-invasively.
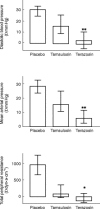
Results: At most time points tamsulosin inhibited phenylephrine-induced diastolic blood pressure elevations significantly less than terazosin (5 h time point: median difference in inhibition 35%, 95% CI: 18.7-50.3%). On the other hand, phenylephrine-induced changes of cardiac output, heart rate and stroke volume were similar during both active treatments.
Conclusions: In doses equi-effective for treatment of lower urinary tract symptoms tamsulosin causes less inhibition of vasoconstriction than terazosin.
Figures


References
-
- van Zwieten PA. α-Adrenoceptor blocking agents in the treatment of hypertension. In: Laragh JH, Brenner BM, editors. Hypertension: Pathophysiology, Diagnosis and Management. 2. New York: Raven Press; 1995. pp. 2917–2935.
-
- Schäfers RF, Poller U, Pönicke K, et al. Influence of adrenoceptor and muscarinic receptor blockade on the cardiovascular effects of exogenous noradrenaline and of endogenous noradrenaline released by infused tyramine. Naunyn-Schmiedeberg’s Arch Pharmacol. 1997;357:239–249. - PubMed
-
- Eri LM, Tveter KJ. α-Blockade in the treatment of symptomatic benign prostatic hyperplasia. J Urol. 1995;154:923–934. - PubMed
-
- Chapple CR. Selective α1-adrenoceptor antagonists in benign prostatic hyperplasia: rationale and clinical experience. Eur Urol. 1996;29:129–144. - PubMed
-
- Kirby RS. Doxazosin in benign prostatic hyperplasia: effects on blood pressure and urinary flow in normotensive and hypertensive men. Urology. 1995;46:182–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

