Effect of mutations in the second extracellular loop of CXCR4 on its utilization by human and feline immunodeficiency viruses
- PMID: 10074102
- PMCID: PMC104012
- DOI: 10.1128/JVI.73.4.2576-2586.1999
Effect of mutations in the second extracellular loop of CXCR4 on its utilization by human and feline immunodeficiency viruses
Abstract
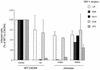
CCR5 and CXCR4 are the principal CD4-associated coreceptors used by human immunodeficiency virus type 1 (HIV-1). CXCR4 is also a receptor for the feline immunodeficiency virus (FIV). The rat CXCR4 cannot mediate infection by HIV-1NDK or by FIVPET (both cell line-adapted strains) because of sequence differences with human CXCR4 in the second extracellular loop (ECL2). Here we made similar observations for HIV-189.6 (a strain also using CCR5) and for a primary HIV-1 isolate. It showed the role of ECL2 in the coreceptor activity of CXCR4 for different types of HIV-1 strains. By exchanging ECL2 residues between human and rat CXCR4, we found that several amino acid differences contributed to the inactivity of the rat CXCR4 toward HIV-189.6. In contrast, its inactivity toward HIV-1NDK seemed principally due to a serine at position 193 instead of to an aspartic acid (Asp193) in human CXCR4. Likewise, a mutation of Asp187 prevented usage of CXCR4 by FIVPET. Different mutations of Asp193, including its replacement by a glutamic acid, markedly reduced or suppressed the activity of CXCR4 for HIV-1NDK infection, indicating that the negative charge was not the only requirement. Mutations of Asp193 and of arginine residues (Arg183 and Arg188) of CXCR4 reduced the efficiency of HIV-1 infection for all HIV-1 strains tested. Other ECL2 mutations tested had strain-specific effects or no apparent effect on HIV-1 infection. The ECL2 mutants allowed us to identify residues contributing to the epitope of the 12G5 monoclonal antibody. Overall, residues with different charges and interspersed in ECL2 seem to participate in the coreceptor activity of CXCR4. This suggests that a conformational rather than linear epitope of ECL2 contributes to the HIV-1 binding site. However, certain HIV-1 and FIV strains seem to require the presence of a particular ECL2 residue.
Figures





References
-
- Bandres J C, Wang Q F, O’Leary J, Baleux F, Amara A, Hoxie J A, Zolla-Pazner S, Gorny M K. Human immunodeficiency virus (HIV) envelope binds to CXCR4 independently of CD4, and binding can be enhanced by interaction with soluble CD4 or by HIV envelope deglycosylation. J Virol. 1998;72:2500–2504. - PMC - PubMed
-
- Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11(Suppl. A):S3–S16. - PubMed
-
- Biti R, Ffrench R, Young J, Bennetts B, Stewart G, Liang T. HIV-1 infection in an individual homozygous for the CCR-5 deletion allele. Nat Med. 1997;3:252–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

