Vif and the p55(Gag) polyprotein of human immunodeficiency virus type 1 are present in colocalizing membrane-free cytoplasmic complexes
- PMID: 10074112
- PMCID: PMC104022
- DOI: 10.1128/JVI.73.4.2667-2674.1999
Vif and the p55(Gag) polyprotein of human immunodeficiency virus type 1 are present in colocalizing membrane-free cytoplasmic complexes
Abstract
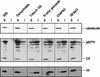
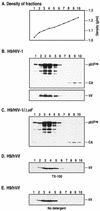
The Vif protein of human immunodeficiency virus type 1 (HIV-1) is a potent regulator of viral infectivity. Current data posit that Vif functions late in replication to modulate assembly, budding, and/or maturation. Consistent with this model, earlier indirect immunofluorescence analyses of HIV-1-infected cells demonstrated that Vif and Gag colocalize to a substantial degree (J. H. M. Simon, R. A. M. Fouchier, T. E. Southerling, C. B. Guerra, C. K. Grant, and M. H. Malim, J. Virol. 71:5259-5267, 1997). Here, we describe a series of subcellular fractionation studies which indicate that Vif and the p55(Gag) polyprotein are present in membrane-free cytoplasmic complexes that copurify in sucrose density gradients and are stable in nonionic detergents. Both Vif and Gag are targeted to these complexes independent of each other, and their association with them appears to be mediated by protein-protein interactions. We propose that these complexes may represent viral assembly intermediates and that Vif is appropriately localized to influence the final stages of the viral life cycle and, therefore, the infectivity of progeny virions.
Figures



References
-
- Bolognesi D P, Montelaro R C, Frank H, Schafer W. Assembly of type C oncornaviruses: a model. Science. 1978;199:183–186. - PubMed
-
- Brown D A, Rose J K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

