Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE
- PMID: 10074118
- PMCID: PMC104028
- DOI: 10.1128/JVI.73.4.2717-2728.1999
Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE
Abstract
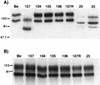
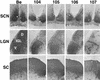
The role of alphaherpesvirus membrane protein internalization during the course of viral infection remains a matter of speculation. To determine the role of internalization of the pseudorabies virus (PRV) gE and gI proteins, we constructed viral mutants encoding specific mutations in the cytoplasmic tail of the gE gene that inhibited internalization of the gE-gI complex. We used these mutants to assess the role of gE-gI endocytosis in incorporation of the proteins into the viral envelope and in gE-mediated spread or gE-promoted virulence. In addition, we report that another viral mutant, PRV 25, which encodes a gE protein defective in endocytosis, contains an additional, previously uncharacterized mutation in the gE gene. We compared PRV 25 to another viral mutant, PRV 107, that does not express the cytoplasmic tail of the gE protein. The gE protein encoded by PRV 107 is also defective in endocytosis. We conclude that efficient endocytosis of gE is not required for gE incorporation into virions, gE-mediated virulence, or spread of virus in the rat central nervous system. However, we do correlate the defect in endocytosis to a small-plaque phenotype in cultured cells.
Figures






Similar articles
-
Role of the pseudorabies virus gI cytoplasmic domain in neuroinvasion, virulence, and posttranslational N-linked glycosylation.J Virol. 2000 Apr;74(8):3505-16. doi: 10.1128/jvi.74.8.3505-3516.2000. J Virol. 2000. PMID: 10729124 Free PMC article.
-
Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI.J Virol. 1996 Apr;70(4):2191-200. doi: 10.1128/JVI.70.4.2191-2200.1996. J Virol. 1996. PMID: 8642642 Free PMC article.
-
Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system.J Virol. 2000 Jan;74(2):834-45. doi: 10.1128/jvi.74.2.834-845.2000. J Virol. 2000. PMID: 10623746 Free PMC article.
-
The role of virion membrane protein endocytosis in the herpesvirus life cycle.J Clin Virol. 2000 Aug;17(2):69-82. doi: 10.1016/s1386-6532(00)00084-6. J Clin Virol. 2000. PMID: 10942087 Review.
-
Glycoprotein E of pseudorabies virus and homologous proteins in other alphaherpesvirinae.Arch Virol. 1994;137(3-4):209-28. doi: 10.1007/BF01309470. Arch Virol. 1994. PMID: 7944945 Review.
Cited by
-
Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network.J Virol. 1999 Nov;73(11):9515-20. doi: 10.1128/JVI.73.11.9515-9520.1999. J Virol. 1999. PMID: 10516060 Free PMC article.
-
Herpes simplex virus gE/gI must accumulate in the trans-Golgi network at early times and then redistribute to cell junctions to promote cell-cell spread.J Virol. 2006 Apr;80(7):3167-79. doi: 10.1128/JVI.80.7.3167-3179.2006. J Virol. 2006. PMID: 16537585 Free PMC article.
-
Expression and characterization of duck enteritis virus gI gene.Virol J. 2011 May 19;8:241. doi: 10.1186/1743-422X-8-241. Virol J. 2011. PMID: 21595918 Free PMC article.
-
EBV BMRF-2 facilitates cell-to-cell spread of virus within polarized oral epithelial cells.Virology. 2009 Jun 5;388(2):335-43. doi: 10.1016/j.virol.2009.03.030. Epub 2009 Apr 24. Virology. 2009. PMID: 19394065 Free PMC article.
-
Deletion of gpUL132, a structural component of human cytomegalovirus, results in impaired virus replication in fibroblasts.J Virol. 2005 Sep;79(18):11837-47. doi: 10.1128/JVI.79.18.11837-11847.2005. J Virol. 2005. PMID: 16140760 Free PMC article.
References
-
- Alconada A, Bauer U, Baudoux L, Piette J, Hoflack B. Intracellular transport of the glycoproteins gE and gI of the varicella-zoster virus. gE accelerates the maturation of gI and determines its accumulation in the trans-Golgi network. J Biol Chem. 1998;273:13430–13436. - PubMed
-
- Audonnet J C, Winslow J, Allen G, Paoletti E. Equine herpesvirus type 1 unique short fragment encodes glycoproteins with homology to herpes simplex virus type 1 gD, gI and gE. J Gen Virol. 1990;71:2969–2978. - PubMed
-
- Babic N, Klupp B, Brack A, Mettenleiter T C, Ugolini G, Flamand A. Deletion of glycoprotein gE reduces the propagation of pseudorabies virus in the nervous system of mice after intranasal inoculation. Virology. 1996;219:279–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

