Absence of internal ribosome entry site-mediated tissue specificity in the translation of a bicistronic transgene
- PMID: 10074119
- PMCID: PMC104029
- DOI: 10.1128/JVI.73.4.2729-2738.1999
Absence of internal ribosome entry site-mediated tissue specificity in the translation of a bicistronic transgene
Abstract
The 5' noncoding regions of the genomes of picornaviruses form a complex structure that directs cap-independent initiation of translation. This structure has been termed the internal ribosome entry site (IRES). The efficiency of translation initiation was shown, in vitro, to be influenced by the binding of cellular factors to the IRES. Hence, we hypothesized that the IRES might control picornavirus tropism. In order to test this possibility, we made a bicistronic construct in which translation of the luciferase gene is controlled by the IRES of Theiler's murine encephalomyelitis virus. In vitro, we observed that the IRES functions in various cell types and in macrophages, irrespective of their activation state. In vivo, we observed that the IRES is functional in different tissues of transgenic mice. Thus, it seems that the IRES is not an essential determinant of Theiler's virus tropism. On the other hand, the age of the mouse could be critical for IRES function. Indeed, the IRES was found to be more efficient in young mice. Picornavirus IRESs are becoming popular tools in transgenesis technology, since they allow the expression of two genes from the same transcription unit. Our results show that the Theiler's virus IRES is functional in cells of different origins and that it is thus a broad-spectrum tool. The possible age dependency of the IRES function, however, could be a drawback for gene expression in adult mice.
Figures


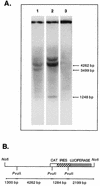

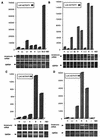
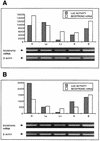
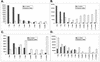
References
-
- Agol V I, Grachev V P, Drosdov S G, Kolesnikova M S, Kozlov V G, Ralph N M, Romanova L I, Tolskaya E A, Tyufanov A V, Viktorova E G. Construction and properties of intertypic poliovirus recombinants: first approximation mapping of the major determinants of neurovirulence. Virology. 1984;136:41–55. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

