Drastic fitness loss in human immunodeficiency virus type 1 upon serial bottleneck events
- PMID: 10074121
- PMCID: PMC104031
- DOI: 10.1128/JVI.73.4.2745-2751.1999
Drastic fitness loss in human immunodeficiency virus type 1 upon serial bottleneck events
Abstract
Muller's ratchet predicts fitness losses in small populations of asexual organisms because of the irreversible accumulation of deleterious mutations and genetic drift. This effect should be enhanced if population bottlenecks intervene and fixation of mutations is not compensated by recombination. To study whether Muller's ratchet could operate in a retrovirus, 10 biological clones were derived from a human immunodeficiency virus type 1 (HIV-1) field isolate by MT-4 plaque assay. Each clone was subjected to 15 plaque-to-plaque passages. Surprisingly, genetic deterioration of viral clones was very drastic, and only 4 of the 10 initial clones were able to produce viable progeny after the serial plaque transfers. Two of the initial clones stopped forming plaques at passage 7, two others stopped at passage 13, and only four of the remaining six clones yielded infectious virus. Of these four, three displayed important fitness losses. Thus, despite virions carrying two copies of genomic RNA and the system displaying frequent recombination, HIV-1 manifested a drastic fitness loss as a result of an accentuation of Muller's ratchet effect.
Figures

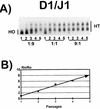


References
-
- Bebeneck K, Kunkel T. The fidelity of retrovial reverse transcriptases. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 85–162.
-
- Chao L. Fitness of RNA virus decreased by Muller’s ratchet. Nature. 1990;348:454–455. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

