The retinoblastoma protein alters the phosphorylation state of polyomavirus large T antigen in murine cell extracts and inhibits polyomavirus origin DNA replication
- PMID: 10074150
- PMCID: PMC104060
- DOI: 10.1128/JVI.73.4.3004-3013.1999
The retinoblastoma protein alters the phosphorylation state of polyomavirus large T antigen in murine cell extracts and inhibits polyomavirus origin DNA replication
Abstract
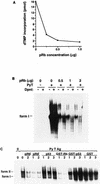
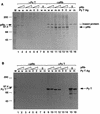
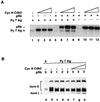
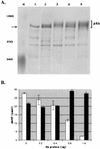
The retinoblastoma tumor suppressor protein (pRb) can associate with the transforming proteins of several DNA tumor viruses, including the large T antigen encoded by polyomavirus (Py T Ag). Although pRb function is critical for regulating progression from G1 to S phase, a role for pRb in S phase has not been demonstrated or excluded. To identify a potential effect of pRb on DNA replication, pRb protein was added to reaction mixtures containing Py T Ag, Py origin-containing DNA (Py ori-DNA), and murine FM3A cell extracts. We found that pRb strongly represses Py ori-DNA replication in vitro. Unexpectedly, however, this inhibition only partially depends on the interaction of pRb with Py T Ag, since a mutant Py T Ag (dl141) lacking the pRb interaction region was also significantly inhibited by pRb. This result suggests that pRb interferes with or alters one or more components of the murine cell replication extract. Furthermore, the ability of Py T Ag to be phosphorylated in such extracts is markedly reduced in the presence of pRb. Since cyclin-dependent kinase (CDK) phosphorylation of Py T Ag is required for its replication function, we hypothesize that pRb interferes with this phosphorylation event. Indeed, the S-phase CDK complex (cyclin A-CDK2), which phosphorylates both pRb and Py T Ag, alleviates inhibition caused by pRb. Moreover, hyperphosphorylated pRb is incapable of inhibiting replication of Py ori-DNA in vitro. We propose a new requirement for maintaining pRb phosphorylation in S phase, namely, to prevent deleterious effects on the cellular replication machinery.
Figures


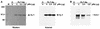
References
-
- Amin A A, Murakami Y, Hurwitz J. Initiation of DNA replication by simian virus 40 T antigen is inhibited by the p107 protein. J Biol Chem. 1994;269:7735–7743. - PubMed
-
- Bártek J, Vojtesek B, Grand R J A, Gallimore P H, Lane D P. Cellular localization and T antigen binding of the retinoblastoma protein. Oncogene. 1992;7:101–108. - PubMed
-
- Bártek J, Bartkova J, Lukas J. The retinoblastoma protein pathway in cell cycle control and cancer. Exp Cell Res. 1997;237:1–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

