Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis
- PMID: 10074164
- PMCID: PMC104074
- DOI: 10.1128/JVI.73.4.3125-3133.1999
Sendai virus and simian virus 5 block activation of interferon-responsive genes: importance for virus pathogenesis
Abstract
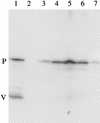
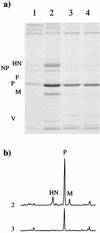
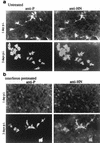
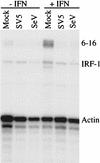
Sendai virus (SeV) is highly pathogenic for mice. In contrast, mice (including SCID mice) infected with simian virus 5 (SV5) showed no overt signs of disease. Evidence is presented that a major factor which prevented SV5 from productively infecting mice was its inability to circumvent the interferon (IFN) response in mice. Thus, in murine cells that produce and respond to IFN, SV5 protein synthesis was rapidly switched off. In marked contrast, once SeV protein synthesis began, it continued, even if the culture medium was supplemented with alpha/beta IFN (IFN-alpha/beta). However, in human cells, IFN-alpha/beta did not inhibit the replication of either SV5 or SeV once virus protein synthesis was established. To begin to address the molecular basis for these observations, the effects of SeV and SV5 infections on the activation of an IFN-alpha/beta-responsive promoter and on that of the IFN-beta promoter were examined in transient transfection experiments. The results demonstrated that (i) SeV, but not SV5, inhibited an IFN-alpha/beta-responsive promoter in murine cells; (ii) both SV5 and SeV inhibited the activation of an IFN-alpha/beta-responsive promoter in human cells; and (iii) in both human and murine cells, SeV was a strong inducer of the IFN-beta promoter, whereas SV5 was a poor inducer. The ability of SeV and SV5 to inhibit the activation of IFN-responsive genes in human cells was confirmed by RNase protection experiments. The importance of these results in terms of paramyxovirus pathogenesis is discussed.
Figures



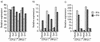

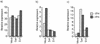
References
-
- Bosma G C, Custer R P, Bosma M J. A severe combined immunodeficiency mutation in mice. Nature. 1983;301:479–482. - PubMed
-
- Choppin P W. Multiplication of a myxovirus (SV5) with minimal cytopathic effects and without interference. Virology. 1964;23:224–233. - PubMed
-
- Darnell J E, Jr, Kerr I M, Stark G R. JAK-STAT pathways and transcriptional activation in response to interferon and other extracellular signalling proteins. Science. 1994;264:1415–1421. - PubMed
-
- Didcock, L., and R. E. Randall. Unpublished data.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

