Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions
- PMID: 10074174
- PMCID: PMC104084
- DOI: 10.1128/JVI.73.4.3210-3218.1999
Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions
Abstract
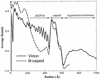
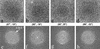
Herpes simplex virus type 1 virions were examined by electron cryomicroscopy, allowing the three-dimensional structure of the infectious particle to be visualized for the first time. The capsid shell is identical to that of B-capsids purified from the host cell nucleus, with the exception of the penton channel, which is closed. The double-stranded DNA genome is organized as regularly spaced ( approximately 26 A) concentric layers inside the capsid. This pattern suggests a spool model for DNA packaging, similar to that for some bacteriophages. The bulk of the tegument is not icosahedrally ordered. However, a small portion appears as filamentous structures around the pentons, interacting extensively with the capsid. Their locations and interactions suggest possible roles for the tegument proteins in regulating DNA transport through the penton channel and binding to cellular transport proteins during viral infection.
Figures





References
-
- Cantor C R, Schimmel P R. Structures of nucleic acids. In: Cantor C R, Schimmel P R, editors. Biophysical chemistry. Part 1. The conformation of biological macromolecules. Vol. 1. San Francisco, Calif: W. H. Freeman and Company; 1980. pp. 155–190.
-
- Cerritelli M E, Cheng N, Rosenberg A H, McPherson C E, Booy F P, Steven A C. Encapsidated conformation of bacteriophage T7 DNA. Cell. 1997;91:271–280. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

