Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells
- PMID: 10074181
- PMCID: PMC104091
- DOI: 10.1128/JVI.73.4.3273-3283.1999
Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells
Abstract
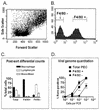
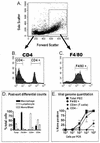
B cells have previously been identified as the major hematopoietic cell type harboring latent gammaherpesvirus 68 (gammaHV68) (N. P. Sunil-Chandra, S. Efstathiou, and A. A. Nash, J. Gen. Virol. 73:3275-3279, 1992). However, we have shown that gammaHV68 efficiently establishes latency in B-cell-deficient mice (K. E. Weck, M. L. Barkon, L. I. Yoo, S. H. Speck, and H. W. Virgin, J. Virol. 70:6775-6780, 1996), demonstrating that B cells are not required for gammaHV68 latency. To understand this dichotomy, we determined whether hematopoietic cell types, in addition to B cells, carry latent gammaHV68. We observed a high frequency of cells that reactivate latent gammaHV68 in peritoneal exudate cells (PECs) derived from both B-cell-deficient and normal C57BL/6 mice. PECs were composed primarily of macrophages in B-cell-deficient mice and of macrophages plus B cells in normal C57BL/6 mice. To determine which cells in PECs from C57BL/6 mice carry latent gammaHV68, we developed a limiting-dilution PCR assay to quantitate the frequency of cells carrying the gammaHV68 genome in fluorescence-activated cell sorter-purified cell populations. We also quantitated the contribution of individual cell populations to the total frequency of cells carrying latent gammaHV68. At early times after infection, the frequency of PECs that reactivated gammaHV68 correlated very closely with the frequency of PECs carrying the gammaHV68 genome, validating measurement of the frequency of viral-genome-positive cells as a measure of latency in this cell population. F4/80-positive macrophage-enriched, lymphocyte-depleted PECs harbored most of the gammaHV68 genome and efficiently reactivated gammaHV68, while CD19-positive, B-cell-enriched PECs harbored about a 10-fold lower frequency of gammaHV68 genome-positive cells. CD4-positive, T-cell-enriched PECs contained only a very low frequency of gammaHV68 genome-positive cells, consistent with previous analyses indicating that T cells are not a reservoir for gammaHV68 latency (N. P. Sunil-Chandra, S. Efstathiou, and A. A. Nash, J. Gen. Virol. 73:3275-3279, 1992). Since macrophages are bone marrow derived, we determined whether elicitation of a large inflammatory response in the peritoneum would recruit additional latent cells into the peritoneum. Thioglycolate inoculation increased the total number of PECs by about 20-fold but did not affect the frequency of cells that reactivate gammaHV68, consistent with a bone marrow reservoir for latent gammaHV68. These experiments demonstrate gammaHV68 latency in two different hematopoietic cell types, F4/80-positive macrophages and CD19-positive B cells, and argue for a bone marrow reservoir for latent gammaHV68.
Figures






References
-
- Austyn J M, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. - PubMed
-
- Bowden R J, Simas J P, Davis A J, Efstathiou S. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J Gen Virol. 1997;78:1675–1687. - PubMed
-
- Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. - PubMed
-
- Cesarman E, Moore P S, Rao P H, Inghirami G, Knowles D M, Chang Y. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi’s sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood. 1995;86:2708–2714. - PubMed
-
- Decker L L, Shankar P, Khan G, Freeman R B, Dezube B J, Lieberman J, Thorley-Lawson D A. The Kaposi’s sarcoma-associated herpesvirus (KSHV) is present as an intact latent genome in KS tissue but replicates in the peripheral blood mononuclear cells of KS patients. J Exp Med. 1996;184:283–288. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

