The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses
- PMID: 10074182
- PMCID: PMC104092
- DOI: 10.1128/JVI.73.4.3284-3291.1999
The U69 gene of human herpesvirus 6 encodes a protein kinase which can confer ganciclovir sensitivity to baculoviruses
Abstract
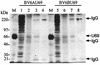
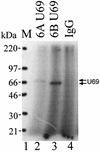
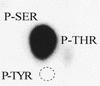
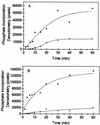
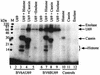
The protein encoded by the U69 open reading frame (ORF) of human herpesvirus 6 (HHV-6) has been predicted to be a protein kinase. To investigate its functional properties, we have expressed the U69 ORFs from both HHV-6 variants, A and B, by using recombinant baculoviruses (BV6AU69 and BV6BU69). Nickel agarose and antibody affinity chromatography was used to purify the proteins to homogeneity and when incubated with [gamma-32P]ATP, both U69 proteins became phosphorylated on predominantly serine residues. These data strongly suggest that U69 is a protein kinase which autophosphorylates. The phosphorylation reaction was optimal at physiological pH and low NaCl concentrations. It required the presence of Mg2+ or Mn2+, and Mg2+ was able to support phosphorylation over a wider range of concentrations than Mn2+. Both ATP and GTP could donate phosphate in the protein kinase assay and the former was more efficient. U69 was capable of phosphorylating histone and casein (serine/threonine kinase substrates) but not enolase (a tyrosine kinase substrate). For the autophosphorylation reaction, the Michaelis constants for ATP of baculovirus-expressed HHV-6A and HHV-6B U69 were calculated to be 44 and 11 microM, respectively. U69 is a homologue of the UL97 gene encoded by human cytomegalovirus which has been shown to phosphorylate the antiviral drug ganciclovir (GCV). We analyzed whether the U69 ORF alone was capable of conferring GCV sensitivity on baculoviruses BV6AU69 and BV6BU69. In plaque reduction experiments, these baculoviruses displayed a GCV-sensitive phenotype compared to a control baculovirus (BVLacZ). The 50% inhibitory concentrations (IC50) of BV6AU69 and BV6BU69 were calculated to be 0.35 and 0.26 mM, respectively, whereas the control baculovirus had an IC50 of >1.4 mM. This shows that the U69 gene product is the only one required to confer GCV sensitivity on baculovirus.
Figures



References
-
- Ablashi D V, Salahuddin S Z, Josephs S F, Imam F, Lusso P, Gallo R C, Hung C, Lemp J, Markham P D. HBLV (or HHV-6) in human cell lines. Nature. 1987;329:207. - PubMed
-
- Agut H, Huraux J M, Collandre H, Montagnier L. Susceptibility of human herpesvirus 6 to acyclovir and ganciclovir. Lancet. 1989;ii:626. - PubMed
-
- Asano Y, Yoshikawa T, Suga S, Yazaki T, Kondo K, Yamanishi K. Fatal fulminant hepatitis in an infant with human herpesvirus-6 infection. Lancet. 1990;335:862–863. - PubMed
-
- Baboonian C, Venables P J, Maini R N, Kangro H O, Osman H K. Antibodies to human herpesvirus-6 in Sjogren’s syndrome. Arthritis Rheum. 1990;33:1749–1750. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

