Effects of human immunodeficiency virus type 1 resistance to protease inhibitors on reverse transcriptase processing, activity, and drug sensitivity
- PMID: 10074202
- PMCID: PMC104112
- DOI: 10.1128/JVI.73.4.3455-3459.1999
Effects of human immunodeficiency virus type 1 resistance to protease inhibitors on reverse transcriptase processing, activity, and drug sensitivity
Abstract
Human immunodeficiency virus type 1 (HIV-1) variants resistant to protease inhibitors often display a reduced replicative capacity as a result of an impairment of protease function. Such fitness-impaired viruses display Gag precursor maturation defects. Here, we report that some protease inhibitor-resistant viruses also display abnormalities in the processing of reverse transcriptase (RT) by the protease. In three recombinant viruses carrying resistant protease sequences from patient plasma, we observed a marked decrease in the amount of mature RT subunits and of particle-associated RT activity compared to their parental pretherapy counterparts. We investigated the possibility that a decrease in the amount of particle-associated mature RT could affect the sensitivity of the corresponding virus to RT inhibitors. We observed a twofold increase of sensitivity to zidovudine (AZT) when a virus which carried AZT mutations was processed by a resistant protease. Interestingly, the presence of AZT-resistance mutations partially rescued the replication defect associated with the mutated protease. The interplay between resistance to protease inhibitors and to RT inhibitors described here may be relevant to the therapeutic control of HIV-1 infection.
Figures

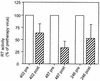
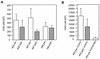

References
-
- Arion D, Borkow G, Kaushik N, Parniak M. Fifth Conference on Retroviruses and Opportunistic Infections, Chicago, Ill. 1998. Phenotypic mechanism of HIV-1 resistance to 3′-azido-2′-deoxythymidine (AZT), abstr. 32.
-
- Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. - PubMed
-
- Barrie K A, Perez E E, Lamers S L, Farmerie W G, Dunn B M, Sleasman J W, Goodenow M M. Natural variation in HIV-1 protease, Gag p7 and p6, and protease cleavage sites within Gag/Pol polyproteins: amino acid substitutions in the absence of protease inhibitors in mothers and children infected by human immunodeficiency virus type 1. Virology. 1996;219:407–416. - PubMed
-
- Borman A, Paulous S, Clavel F. Resistance of HIV-1 to protease inhibitors: selection of resistance mutations in the presence and in the absence of the drug. J Gen Virol. 1996;77:419–426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

