Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle's syndrome
- PMID: 10074483
- PMCID: PMC408130
- DOI: 10.1172/JCI5713
Defective regulation of the epithelial Na+ channel by Nedd4 in Liddle's syndrome
Abstract
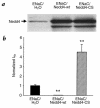
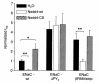
Liddle's syndrome is an inherited form of hypertension linked to mutations in the epithelial Na+ channel (ENaC). ENaC is composed of three subunits (alpha, beta, gamma), each containing a COOH-terminal PY motif (xPPxY). Mutations causing Liddle's syndrome alter or delete the PY motifs of beta- or gamma-ENaC. We recently demonstrated that the ubiquitin-protein ligase Nedd4 binds these PY motifs and that ENaC is regulated by ubiquitination. Here, we investigate, using the Xenopus oocyte system, whether Nedd4 affects ENaC function. Overexpression of wild-type Nedd4, together with ENaC, inhibited channel activity, whereas a catalytically inactive Nedd4 stimulated it, likely by acting as a competitive antagonist to endogenous Nedd4. These effects were dependant on the PY motifs, because no Nedd4-mediated changes in channel activity were observed in ENaC lacking them. The effect of Nedd4 on ENaC missing only one PY motif (of beta-ENaC), as originally described in patients with Liddle's syndrome, was intermediate. Changes were due entirely to alterations in ENaC numbers at the plasma membrane, as determined by surface binding and immunofluorescence. Our results demonstrate that Nedd4 is a negative regulator of ENaC and suggest that the loss of Nedd4 binding sites in ENaC observed in Liddle's syndrome may explain the increase in channel number at the cell surface, increased Na+ reabsorption by the distal nephron, and hence the hypertension.
Figures



References
-
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. - PubMed
-
- Shimkets RA, et al. Liddle's syndrome: heritable human hypertension caused by mutations in the β subunit of the epithelial sodium channel. Cell. 1994;79:407–414. - PubMed
-
- Chang SS, et al. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat Genet. 1996;12:248–253. - PubMed
-
- Stutts MJ, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. - PubMed
-
- Hummler E, et al. Early death due to defective neonatal lung liquid clearance in αENaC-deficient mice. Nat Genet. 1996;12:325–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

