Impairment of skeletal muscle adenosine triphosphate-sensitive K+ channels in patients with hypokalemic periodic paralysis
- PMID: 10074484
- PMCID: PMC408119
- DOI: 10.1172/JCI4552
Impairment of skeletal muscle adenosine triphosphate-sensitive K+ channels in patients with hypokalemic periodic paralysis
Abstract
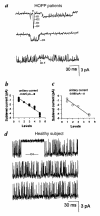
The adenosine triphosphate (ATP)-sensitive K+ (KATP) channel is the most abundant K+ channel active in the skeletal muscle fibers of humans and animals. In the present work, we demonstrate the involvement of the muscular KATP channel in a skeletal muscle disorder known as hypokalemic periodic paralysis (HOPP), which is caused by mutations of the dihydropyridine receptor of the Ca2+ channel. Muscle biopsies excised from three patients with HOPP carrying the R528H mutation of the dihydropyridine receptor showed a reduced sarcolemma KATP current that was not stimulated by magnesium adenosine diphosphate (MgADP; 50-100 microM) and was partially restored by cromakalim. In contrast, large KATP currents stimulated by MgADP were recorded in the healthy subjects. At channel level, an abnormal KATP channel showing several subconductance states was detected in the patients with HOPP. None of these were surveyed in the healthy subjects. Transitions of the KATP channel between subconductance states were also observed after in vitro incubation of the rat muscle with low-K+ solution. The lack of the sarcolemma KATP current observed in these patients explains the symptoms of the disease, i.e., hypokalemia, depolarization of the fibers, and possibly the paralysis following insulin administration.
Figures
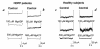



References
-
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. - PubMed
-
- Sakura H, Ämmälä C, Smith PA, Gribble FM, Ashcroft FM. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. - PubMed
-
- Tricarico D, Conte Camerino D. ATP-sensitive K+ channels of skeletal muscle fibers from young adult and aged rats: possible involvement of thiol-dependent redox mechanisms in the age-dependent modifications of their biophysical and pharmacological properties. Mol Pharmacol. 1994;46:754–761. - PubMed
-
- Tricarico D, Petruzzi R, Conte Camerino D. Different sulfonylurea and ATP sensitivity characterizes the juvenile and the adult form of KATP channel complex of rat skeletal muscle. Eur J Pharmacol. 1997;321:369–378. - PubMed
-
- Tricarico D, et al. The biophysical and pharmacological characteristics of skeletal muscle KATP channels are modified in K+ depleted rat, an animal model of hypokalemic periodic paralysis. Mol Pharmacol. 1998;54:197–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

