Hypertension in mice lacking 11beta-hydroxysteroid dehydrogenase type 2
- PMID: 10074485
- PMCID: PMC408118
- DOI: 10.1172/JCI4445
Hypertension in mice lacking 11beta-hydroxysteroid dehydrogenase type 2
Abstract
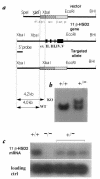
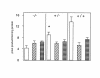
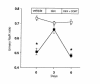
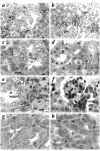
Deficiency of 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) in humans leads to the syndrome of apparent mineralocorticoid excess (SAME), in which cortisol illicitly occupies mineralocorticoid receptors, causing sodium retention, hypokalemia, and hypertension. However, the disorder is usually incompletely corrected by suppression of cortisol, suggesting additional and irreversible changes, perhaps in the kidney. To examine this further, we produced mice with targeted disruption of the 11beta-HSD2 gene. Homozygous mutant mice (11beta-HSD2(-/-)) appear normal at birth, but approximately 50% show motor weakness and die within 48 hours. Both male and female survivors are fertile but exhibit hypokalemia, hypotonic polyuria, and apparent mineralocorticoid activity of corticosterone. Young adult 11beta-HSD2(-/-) mice are markedly hypertensive, with a mean arterial blood pressure of 146 +/- 2 mmHg, compared with 121 +/- 2 mmHg in wild-type controls and 114 +/- 4 mmHg in heterozygotes. The epithelium of the distal tubule of the nephron shows striking hypertrophy and hyperplasia. These histological changes do not readily reverse with mineralocorticoid receptor antagonism in adulthood. Thus, 11beta-HSD2(-/-) mice demonstrate the major features of SAME, providing a unique rodent model to study the molecular mechanisms of kidney resetting leading to hypertension.
Figures
References
-
- Guyton AC. Blood pressure control—special role of the kidneys and body fluids. Science. 1991;252:1813–1816. - PubMed
-
- Shimkets RA, et al. Liddle's syndrome: heritable human hypertension caused by mutations in the beta subunit of the epithelial sodium channel. Cell. 1994;79:407–414. - PubMed
-
- Simon DB, et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet. 1997;17:171–178. - PubMed
-
- Lifton RP, et al. A chimaeric 11β-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

