Cardiomyocytes can be generated from marrow stromal cells in vitro
- PMID: 10074487
- PMCID: PMC408125
- DOI: 10.1172/JCI5298
Cardiomyocytes can be generated from marrow stromal cells in vitro
Abstract
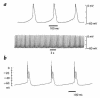
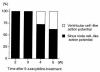
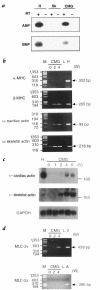
We have isolated a cardiomyogenic cell line (CMG) from murine bone marrow stromal cells. Stromal cells were immortalized, treated with 5-azacytidine, and spontaneously beating cells were repeatedly screened. The cells showed a fibroblast-like morphology, but the morphology changed after 5-azacytidine treatment in approximately 30% of the cells; they connected with adjoining cells after one week, formed myotube-like structures, began spontaneously beating after two weeks, and beat synchronously after three weeks. They expressed atrial natriuretic peptide and brain natriuretic peptide and were stained with anti-myosin, anti-desmin, and anti-actinin antibodies. Electron microscopy revealed a cardiomyocyte-like ultrastructure, including typical sarcomeres, a centrally positioned nucleus, and atrial granules. These cells had several types of action potentials, such as sinus node-like and ventricular cell-like action potentials. All cells had a long action potential duration or plateau, a relatively shallow resting membrane potential, and a pacemaker-like late diastolic slow depolarization. Analysis of the isoform of contractile protein genes, such as myosin heavy chain, myosin light chain, and alpha-actin, indicated that their muscle phenotype was similar to that of fetal ventricular cardiomyocytes. These cells expressed Nkx2.5/Csx, GATA4, TEF-1, and MEF-2C mRNA before 5-azacytidine treatment and expressed MEF-2A and MEF-2D after treatment. This new cell line provides a powerful model for the study of cardiomyocyte differentiation.
Figures






References
-
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. - PubMed
-
- Silberstein L, Webster SG, Travis M, Blau HM. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 1986;46:1075–1081. - PubMed
-
- Olson EN, Srivastava D. Molecular pathways controlling heart development. Science. 1996;272:671–676. - PubMed
-
- Lassar AB, Paterson BM, Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T1/2 fibroblasts to myoblasts. Cell. 1986;47:649–656. - PubMed
-
- Taylor SM, Jones PA. Multiple new phenotypes induced in 10T1/2 and 3T3 cells treated with 5-azacytidine. Cell. 1979;17:771–779. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

