Rapid and sensitive detection of immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay
- PMID: 10074525
- PMCID: PMC88648
- DOI: 10.1128/JCM.37.4.1049-1056.1999
Rapid and sensitive detection of immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay
Abstract
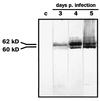
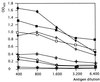
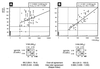
Canine distemper morbillivirus (CDV) infection causes a frequently fatal systemic disease in a broad range of carnivore species, including domestic dogs. In CDV infection, classical serology provides data of diagnostic and prognostic values (kinetics of seroconversion) and is also used to predict the optimal vaccination age of pups. Routine CDV serology is still based on time- and cost-intensive virus neutralization assays (V-NA). Here, we describe a new capture-sandwich enzyme-linked immunosorbent assay (ELISA) that uses recombinant baculovirus-expressed nucleocapsid (N) protein of a recent CDV wild-type isolate (2544/Han95) for the detection of CDV-specific antibodies in canine sera. Recombinant antigen was produced with high efficacy in Heliothis virescens larvae. The capture-sandwich ELISA enabled a clear-cut qualitative evaluation of the CDV-specific immunoglobulin G (IgG) and IgM serostatuses of 196 and 35 dog sera, respectively. Inter-rater agreement analysis (kappa = 0.988) indicated that the ELISA can be used unrestrictedly as a substitute for the V-NA for the qualitative determination of CDV-specific IgG serostatus. In an attempt to semiquantify N-specific antibodies, a one-step-dilution (alpha method) IgG-specific ELISA was implemented. Alpha values of >/=50% showed very good inter-rater agreement (kappa = 0.968) with V-NA titers of >/=1/100 50% neutralizing dose (ND50) as measured against the central European CDV wild-type isolate 2544/Han95 in canine sera originating from northern Germany. An ND50 titer of 1/100 is considered a threshold, and titers of >/=1/100 indicate a resilient, protective immunity. CDV N-specific antibodies of the IgM class were detected by the newly developed ELISA in 9 of 15 sera obtained from dogs with symptoms of acute distemper. In leucocytes of 5 of the 15 dogs (all of which were also IgM positive) CDV RNA was detected by reverse transcription (RT)-PCR. The recombinant capture-sandwich ELISA detecting N-specific antibodies of the IgG class provided superior sensitivity and specificity and thus represents a rapid and cost-effective alternative to classical CDV V-NA. By detection of specific IgM antibodies, the ELISA will be complementary to RT-PCR and V-NA in the diagnosis of acute distemper infections.
Figures

Similar articles
-
Detection of IgM antibodies against a recombinant nucleocapsid protein of canine distemper virus in dog sera using a dot-blot assay.Zentralbl Veterinarmed A. 1999 Mar;46(2):115-21. doi: 10.1046/j.1439-0442.1999.00198.x. Zentralbl Veterinarmed A. 1999. PMID: 10216448
-
Antigen requirements and specificity of enzyme-linked immunosorbent assay for detection of canine IgG against canine distemper viral antigens.Am J Vet Res. 1982 Dec;43(12):2266-9. Am J Vet Res. 1982. PMID: 6187248
-
Detection of IgM antibodies against canine distemper virus in dog and mink sera employing enzyme-linked immunosorbent assay (ELISA).J Vet Diagn Invest. 1991 Jan;3(1):3-9. doi: 10.1177/104063879100300102. J Vet Diagn Invest. 1991. PMID: 2039785
-
Canine distemper infections, with special reference to South Africa, with a review of the literature.J S Afr Vet Assoc. 2001 Sep;72(3):127-36. doi: 10.4102/jsava.v72i3.635. J S Afr Vet Assoc. 2001. PMID: 11811699 Review.
-
Safety and Immunogenicity of Morbillivirus canis Vaccines for Domestic and Wild Animals: A Scoping Review.Viruses. 2024 Jul 4;16(7):1078. doi: 10.3390/v16071078. Viruses. 2024. PMID: 39066240 Free PMC article.
Cited by
-
Infection with a Mouse-Adapted Strain of the 2009 Pandemic Virus Causes a Highly Severe Disease Associated with an Impaired T Cell Response.PLoS One. 2015 Sep 18;10(9):e0138055. doi: 10.1371/journal.pone.0138055. eCollection 2015. PLoS One. 2015. PMID: 26381265 Free PMC article.
-
Canine Distemper Virus: Origins, Mutations, Diagnosis, and Epidemiology in Mexico.Life (Basel). 2024 Aug 13;14(8):1002. doi: 10.3390/life14081002. Life (Basel). 2024. PMID: 39202744 Free PMC article. Review.
-
Development of a duplex real-time RT-qPCR assay to monitor genome replication, gene expression and gene insert stability during in vivo replication of a prototype live attenuated canine distemper virus vector encoding SIV gag.J Virol Methods. 2015 Mar;213:26-37. doi: 10.1016/j.jviromet.2014.11.015. Epub 2014 Dec 5. J Virol Methods. 2015. PMID: 25486083 Free PMC article.
-
Rapid and sensitive detection of canine distemper virus by one-tube reverse transcription-insulated isothermal polymerase chain reaction.BMC Vet Res. 2014 Sep 9;10:213. doi: 10.1186/s12917-014-0213-8. BMC Vet Res. 2014. PMID: 25200113 Free PMC article.
-
Inactivated Recombinant Rabies Viruses Displaying Canine Distemper Virus Glycoproteins Induce Protective Immunity against Both Pathogens.J Virol. 2017 Mar 29;91(8):e02077-16. doi: 10.1128/JVI.02077-16. Print 2017 Apr 15. J Virol. 2017. PMID: 28148801 Free PMC article.
References
-
- Appel M J G, Gillespie J H. Canine distemper virus. Virol Monogr. 1972;11:1–96.
-
- Appel M J G, Robson D S. A microneutralization test for canine distemper virus. Am J Vet Res. 1973;34:1459–1463. - PubMed
-
- Appel M J G, Shek W R, Sheshberadaran H, Norrby E. Measles virus and inactivated canine distemper virus induce incomplete immunity to canine distemper. Arch Virol. 1984;82:73–82. - PubMed
-
- Appel M G J, Yates R A, Foley G L, Bernstein J J, Santinelli S, Spelman L H, Miller L D, Arp L H, Anderson M, Barr M, Pearce-Kelling S, Summers B A. Canine distemper epizootic in lions, tigers, and leopards in North America. J Vet Diagn Investig. 1994;6:277–288. - PubMed
-
- Blixenkrone-Möller M, Pedersen I R, Appel M J, Griot C. Detection of IgM antibodies against canine distemper virus in dog and mink sera employing enzyme-linked immunosorbent assay (ELISA) J Vet Diagn Investig. 1991;3:3–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

