Bacteroides fragilis toxin 2 damages human colonic mucosa in vitro
- PMID: 10075957
- PMCID: PMC1727476
- DOI: 10.1136/gut.44.4.504
Bacteroides fragilis toxin 2 damages human colonic mucosa in vitro
Abstract
Background: Strains of Bacteroides fragilis producing a 20 kDa protein toxin (B fragilis toxin (BFT) or fragilysin) are associated with diarrhoea in animals and humans. Although in vitro results indicate that BFT damages intestinal epithelial cells in culture, the effects of BFT on native human colon are not known.
Aims: To examine the electrophysiological and morphological effects of purified BFT-2 on human colonic mucosa in vitro.
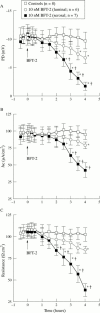
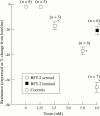
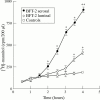
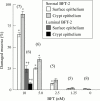
Methods: For resistance (R) measurements, colonic mucosa mounted in Ussing chambers was exposed to luminal or serosal BFT-2 (1.25-10 nM) and after four hours morphological damage was measured on haematoxylin and eosin stained sections using morphometry. F actin distribution was assessed using confocal microscopy.
Results: Serosal BFT-2 for four hours was four-, two-, seven-, and threefold more potent than luminal BFT-2 in decreasing resistance, increasing epithelial 3H-mannitol permeability, and damaging crypt and surface colonocytes, respectively (p<0.05). Confocal microscopy showed reduced colonocyte F actin staining intensity after exposure to BFT-2.
Conclusions: BFT-2 increases human colonic permeability and damages human colonic epithelial cells in vitro. These effects may be important in the development of diarrhoea and intestinal inflammation caused by B fragilis in vivo.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
