Recognition of the major histocompatibility complex restriction element modulates CD8(+) T cell specificity and compensates for loss of T cell receptor contacts with the specific peptide
- PMID: 10075972
- PMCID: PMC2193044
- DOI: 10.1084/jem.189.6.883
Recognition of the major histocompatibility complex restriction element modulates CD8(+) T cell specificity and compensates for loss of T cell receptor contacts with the specific peptide
Abstract
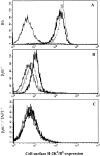
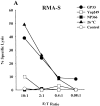
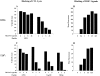
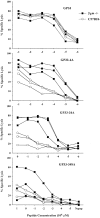
Triggering of a T cell requires interaction between its specific receptor (TCR) and a peptide antigen presented by a self-major histocompatibility complex (MHC) molecule. TCR recognition of self-MHC by itself falls below the threshold of detection in most systems due to low affinity. To study this interaction, we have used a read-out system in which antigen-specific effector T cells are confronted with targets expressing high levels of MHC compared with the selecting and priming environment. More specifically, the system is based on CD8(+) T cells selected in an environment with subnormal levels of MHC class I in the absence of beta2-microglobulin. We observe that the MHC restriction element can trigger viral peptide-specific T cells independently of the peptide ligand, provided there is an increase in self-MHC density. Peptide-independent triggering required at least four times the natural in vivo level of MHC expression. Furthermore, recognition of the restriction element at expression levels below this threshold was still enough to compensate for lack of affinity to peptides carrying alanine substitutions in major TCR contact residues. Thus, the specificity in TCR recognition and T cell activation is fine tuned by the avidity for self-MHC, and TCR avidities for peptide and MHC may substitute for each other. These results demonstrate a functional role for TCR avidity for self-MHC in tuning of T cell specificity, and support a role for cross-reactivity on "self" during T cell selection and activation.
Figures




Similar articles
-
Effective adoptive therapy of tap-deficient lymphoma using diverse high avidity alloreactive T cells.Cancer Immunol Immunother. 2010 Apr;59(4):629-33. doi: 10.1007/s00262-009-0805-5. Epub 2009 Dec 18. Cancer Immunol Immunother. 2010. PMID: 20020123 Free PMC article.
-
Discrepancy between in vitro measurable and in vivo virus neutralizing cytotoxic T cell reactivities. Low T cell receptor specificity and avidity sufficient for in vitro proliferation or cytotoxicity to peptide-coated target cells but not for in vivo protection.J Immunol. 1992 Aug 1;149(3):972-80. J Immunol. 1992. PMID: 1634779
-
A low affinity TCR ligand restores positive selection of CD8+ T cells in vivo.J Immunol. 2001 Jun 1;166(11):6602-7. doi: 10.4049/jimmunol.166.11.6602. J Immunol. 2001. PMID: 11359813
-
Co-receptors and recognition of self at the immunological synapse.Curr Top Microbiol Immunol. 2010;340:171-89. doi: 10.1007/978-3-642-03858-7_9. Curr Top Microbiol Immunol. 2010. PMID: 19960314 Free PMC article. Review.
-
Peptide-dependent tuning of major histocompatibility complex motional properties and the consequences for cellular immunity.Curr Opin Immunol. 2022 Jun;76:102184. doi: 10.1016/j.coi.2022.102184. Epub 2022 May 9. Curr Opin Immunol. 2022. PMID: 35550277 Free PMC article. Review.
Cited by
-
TCR transgenes and transgene cassettes for TCR gene therapy: status in 2008.Cancer Immunol Immunother. 2009 May;58(5):809-22. doi: 10.1007/s00262-008-0649-4. Epub 2009 Feb 3. Cancer Immunol Immunother. 2009. PMID: 19189103 Free PMC article. Review.
-
High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines.J Immunol. 2007 Nov 1;179(9):5845-54. doi: 10.4049/jimmunol.179.9.5845. J Immunol. 2007. PMID: 17947658 Free PMC article.
-
Chronic TCR-MHC (self)-interactions limit the functional potential of TCR affinity-increased CD8 T lymphocytes.J Immunother Cancer. 2019 Nov 5;7(1):284. doi: 10.1186/s40425-019-0773-z. J Immunother Cancer. 2019. PMID: 31690351 Free PMC article.
-
Effective adoptive therapy of tap-deficient lymphoma using diverse high avidity alloreactive T cells.Cancer Immunol Immunother. 2010 Apr;59(4):629-33. doi: 10.1007/s00262-009-0805-5. Epub 2009 Dec 18. Cancer Immunol Immunother. 2010. PMID: 20020123 Free PMC article.
References
-
- Zinkernagel RM, Doherty PC. Restriction of in vitro T cell-mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. - PubMed
-
- Bjorkman PJ. MHC restriction in three dimensions: a view of T cell receptor/ligand interactions. Cell. 1997;89:167–170. - PubMed
-
- Blackman M, Yague J, Kubo R, Gay D, Coleclough C, Palmer E, Kappler J, Marrack P. The T cell repertoire may be biased in favor of MHC recognition. Cell. 1986;47:349–357. - PubMed
-
- Jerne N. The somatic generation of antibody diversity. Eur J Immunol. 1971;1:1–9. - PubMed
-
- Zerrahn J, Held W, Raulet DH. The MHC reactivity of the T cell repertoire prior to positive and negative selection. Cell. 1997;88:627–636. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

