Characterization of the 4C8 antigen involved in transendothelial migration of CD26(hi) T cells after tight adhesion to human umbilical vein endothelial cell monolayers
- PMID: 10075981
- PMCID: PMC2193050
- DOI: 10.1084/jem.189.6.979
Characterization of the 4C8 antigen involved in transendothelial migration of CD26(hi) T cells after tight adhesion to human umbilical vein endothelial cell monolayers
Abstract
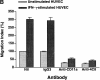
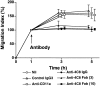
In extravasation of T cells, little is known about the mechanisms of transendothelial migration subsequent to the T cells' tight adhesion to endothelium. To investigate these mechanisms, we developed a monoclonal antibody (mAb), termed anti-4C8, that blocks transmigration but not adhesion in a culture system in which high CD26-expressing (CD26(hi)) T cells preferentially migrate through human umbilical vein endothelial cell (HUVEC) monolayers cultured on collagen gels. Anti-4C8 reacted with all CD3(+) T cells and monocytes but not neutrophils or HUVECs. The structure defined by this antibody was an 80-kD molecule. The mAb at 1 mug/ml inhibited 80-90% of migration of CD3(+) T cells through unstimulated and interferon gamma-stimulated HUVEC monolayers without interfering with adhesion and cell motility. When added to the cultures after the adhesion, anti-4C8 completely blocked subsequent transmigration of adherent T cells. Phase-contrast and electron microscopy revealed that T cells are arrested at the intercellular junctions of HUVECs in the presence of anti-4C8. Anti-4C8 exhibited agonistic effects on resting T cells without other stimuli under culture conditions in which anti-4C8 can stimulate T cells. First, in the checkerboard assay using collagen gels, the antibody promoted chemokinetic migration of the cells in a dose-dependent manner from 0.1 to 10 mug/ml. The predominant population of T cells that migrated into collagen gels with impregnated anti-4C8 were CD26(hi). Second, solid-phase-immobilized anti-4C8 induced adhesion of T cells to the substrate, often with polarizations in cell shape and large pseudopods rich in filamentous (F-) actin. Third, soluble anti-4C8 augmented F-actin content preferentially in CD26(hi) T cells when added to T cells at a high dose of 10 mug/ml. Finally, both anti-4C8-induced chemokinetic migration and transendothelial migration were inhibited by pretreatment of T cells with pertussis toxin. These findings suggest that stimulation via the 4C8 antigen increases cell motility of CD26(hi) cells with profound cytoskeletal changes through signaling pathways including G proteins. The 4C8 antigen may be involved in preferential transmigration of CD26(hi) cells adherent to HUVECs.
Figures













References
-
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
-
- Bianchi E, Bender JR, Blasi F, Pardi R. Through and beyond the wall: late steps in leukocyte transendothelial migration. Immunol Today. 1997;18:586–591. - PubMed
-
- Muller WA. The role of PECAM-1 (CD31) in leukocyte emigration: studies in vitro and in vivo. J Leukoc Biol. 1995;57:523–528. - PubMed
-
- Allport JR, Ding H, Collins T, Gerristen ME, Luscinskas FW. Endothelial-dependent mechanisms regulate leukocyte transmigration: a process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J Exp Med. 1997;186:517–527. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

