Resistance of CD7-deficient mice to lipopolysaccharide-induced shock syndromes
- PMID: 10075985
- PMCID: PMC2193045
- DOI: 10.1084/jem.189.6.1011
Resistance of CD7-deficient mice to lipopolysaccharide-induced shock syndromes
Abstract
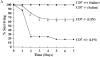
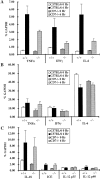
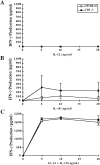
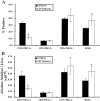
CD7 is an immunoglobulin superfamily molecule involved in T and natural killer (NK) cell activation and cytokine production. CD7-deficient animals develop normally but have antigen-specific defects in interferon (IFN)-gamma production and CD8(+) CTL generation. To determine the in vivo role of CD7 in systems dependent on IFN-gamma, the response of CD7-deficient mice to lipopolysaccharide (LPS)-induced shock syndromes was studied. In the high-dose LPS-induced shock model, 67% of CD7-deficient mice survived LPS injection, whereas 19% of control C57BL/6 mice survived LPS challenge (P < 0.001). CD7-deficient or C57BL/6 control mice were next injected with low-dose LPS (1 microgram plus 8 mg D-galactosamine [D-gal] per mouse) and monitored for survival. All CD7-deficient mice were alive 72 h after injection of LPS compared with 20% of C57BL/6 control mice (P < 0.001). After injection of LPS and D-gal, CD7-deficient mice had decreased serum IFN-gamma and tumor necrosis factor (TNF)-alpha levels compared with control C57BL/6 mice (P < 0.001). Steady-state mRNA levels for IFN-gamma and TNF-alpha in liver tissue were also significantly decreased in CD7-deficient mice compared with controls (P < 0.05). In contrast, CD7-deficient animals had normal liver interleukin (IL)-12, IL-18, and interleukin 1 converting enzyme (ICE) mRNA levels, and CD7-deficient splenocytes had normal IFN-gamma responses when stimulated with IL-12 and IL-18 in vitro. NK1.1(+)/ CD3(+) T cells are known to be key effector cells in the pathogenesis of toxic shock. Phenotypic analysis of liver mononuclear cells revealed that CD7-deficient mice had fewer numbers of liver NK1.1(+)/CD3(+) T cells (1.5 +/- 0.3 x 10(5)) versus C57BL/6 control mice (3.7 +/- 0.8 x 10(5); P < 0.05), whereas numbers of liver NK1.1(+)/CD3(-) NK cells were not different from controls. Thus, targeted disruption of CD7 leads to a selective deficiency of liver NK1.1(+)/ CD3(+) T cells, and is associated with resistance to LPS shock. These data suggest that CD7 is a key molecule in the inflammatory response leading to LPS-induced shock.
Figures


References
-
- Haynes BF, Denning SM, Le PT, Singer KH. Human intrathymic T cell differentiation. Semin Immunol. 1990;2:67–77. - PubMed
-
- Chabannon C, Wood P, Torok-Storb B. Expression of CD7 on normal human myeloid progenitors. J Immunol. 1992;149:2110–2113. - PubMed
-
- Barcena A, Muench MO, Galy AH, Cupp J, Roncarolo MG, Phillips JH, Spits H. Phenotypic and functional analysis of T-cell precursors in the human fetal liver and thymus: CD7 expression in the early stages of T- and myeloid-cell development. Blood. 1993;82:3401–3414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

