Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin
- PMID: 10077570
- PMCID: PMC15828
- DOI: 10.1073/pnas.96.6.2678
Lysozyme and RNases as anti-HIV components in beta-core preparations of human chorionic gonadotropin
Abstract
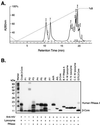
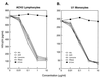
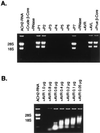
Human chorionic gonadotropin (hCG) preparations contain activity against HIV type 1 (HIV-1). However, there has been controversy about whether some biological activities of hCG beta-subunit (hCGbeta) preparations are caused by the beta-subunit itself or other proteins present in the preparations. We report here the purification, characterization, and identification of three enzymes with anti-HIV activity present in the beta-core fraction of hCGbeta prepared from the urine of pregnant women. The N-terminal amino acid sequence of one protein is identical to human urinary lysozyme C, and those of the other two are identical to human RNase A and urinary RNase U. We thus refer to these proteins as AVL (antiviral lysozyme) and AVR (antiviral RNases). In addition to HIV-1 inhibition, AVL is capable of lysing Micrococcus lysodeikticus. AVR digests a variety of RNA substrates, including RNA from HIV-1-infected cells. We also find that lysozyme from chicken egg white, human milk, and human neutrophils and RNase A from bovine pancreas possess activity against HIV-1. These findings may offer additional strategies for the treatment of HIV-1 infection.
Figures
References
-
- De Rossi A, Ometto L, Mammano F, Zanotto C, Giaquinto C, Chieco-Bianchi L. AIDS. 1992;6:1117–1120. - PubMed
-
- Krivine A, Firtion G, Cao L, Francoual C, Henrion R, Lebon P. Lancet. 1992;339:1187–1189. - PubMed
-
- Bourinbaiar A S, Lee-Huang S. Immunol Lett. 1995;44:13–18. - PubMed
-
- Lunardi-Iskandar Y, Bryant J L, Zeman R A, Lam V H, Samaniego F, Besnier J M, Hermans P, Thierry A R, Gill P, Gallo R C. Nature (London) 1995;375:64–68. - PubMed
-
- Gill P S, Lunardi-Ishkandar Y, Louie S, Tulpule A, Zheng T, Espina B M, Besnier J M, Hermans P, Levine A M, Bryant J L, Gallo R C. N Engl J Med. 1996;335:1261–1269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

