Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function
- PMID: 10077597
- PMCID: PMC15855
- DOI: 10.1073/pnas.96.6.2834
Intermolecular interactions between dimeric calcium-sensing receptor monomers are important for its normal function
Abstract
We recently demonstrated that the G protein-coupled, extracellular calcium-sensing receptor (CaR) forms disulfide-linked dimers. The functional significance of dimerization of this receptor was suggested by our earlier observations that CaRs carrying certain point mutations exert dominant negative effects on the function of the coexpressed wild-type receptor both in vivo and when cotransfected in human embryonic kidney cells. In this study, we explored the functional consequences of CaR dimerization. Coexpression in human embryonic kidney cells of specific pairs of mutant CaRs, each with reduced or absent activity because of distinct loss-of-function mutations, results in the formation of heterodimers and partially reconstitutes extracellular calcium-dependent signaling. Moreover, our results suggest that the CaR has at least two functionally separable domains. However, the presence of an abnormal domain in each mutant monomer substantially impairs the function of the CaR heterodimer, resulting in the reconstituted CaRs having characteristics distinct from those of the wild-type CaR. Our study suggests that intermolecular interactions within the dimeric CaR are important for the receptor's function.
Figures

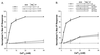

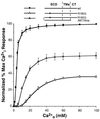


References
-
- Brown E M, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger M A, Lytton J, Hebert S C. Nature (London) 1993;366:575–580. - PubMed
-
- Ward D T, Brown E M, Harris H W. J Biol Chem. 1998;273:14476–14483. - PubMed
-
- Bai M, Trivedi S, Brown E M. J Biol Chem. 1998;273:23605–23610. - PubMed
-
- Pollak M R, Brown E M, Chou Y H, Hebert S C, Marx S J, Steinmann B, Levi T, Seidman C E, Seidman J G. Cell. 1993;75:1297–1303. - PubMed
-
- Pollak M R, Brown E M, Estep H L, McLaine P N, Kifor O, Park J, Hebert S C, Seidman C E, Seidman J G. Nat Genet. 1994;8:303–307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

