Cyclophilin C-associated protein: a normal secreted glycoprotein that down-modulates endotoxin and proinflammatory responses in vivo
- PMID: 10077627
- PMCID: PMC15885
- DOI: 10.1073/pnas.96.6.3006
Cyclophilin C-associated protein: a normal secreted glycoprotein that down-modulates endotoxin and proinflammatory responses in vivo
Abstract
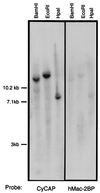
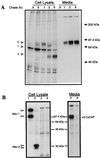
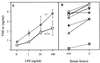
Mouse cyclophilin C-associated protein (CyCAP) is a member of the scavenger-receptor cysteine-rich domain superfamily and is 69% identical to the human Mac-2 binding protein. Here, we show that CyCAP is a widely expressed secreted glycoprotein that modulates the host response to endotoxin. Gene-targeted CyCAP-deficient mice are more sensitive to the lethal effects of endotoxin. In response to endotoxin, CyCAP-deficient mice overproduced interleukin 12 and interferon-gamma systemically and tumor necrosis factor alpha locally; these are proinflammatory molecules that also promote T helper 1 responses. Furthermore, macrophages stimulated in vitro with endotoxin in serum deficient in CyCAP secreted more tumor necrosis factor alpha, supporting the proposal that CyCAP specifically down-modulates endotoxin signaling.
Figures




References
-
- Friedman J, Weissman I. Cell. 1991;66:799–806. - PubMed
-
- Chicheportiche Y, Vassalli P. J Biol Chem. 1994;269:5512–5517. - PubMed
-
- Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Nature (London) 1990;343:531–535. - PubMed
-
- Resnick D, Pearson A, Krieger M. Trends Biochem Sci. 1994;19:5–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

