In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells
- PMID: 10077631
- PMCID: PMC15889
- DOI: 10.1073/pnas.96.6.3029
In vitro comparison of the antigen-binding and stability properties of the various molecular forms of IgA antibodies assembled and produced in CHO cells
Abstract
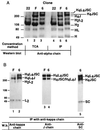
The hallmark of a mucosal immune response is the production of antigen-specific secretory IgA (S-IgA) antibodies in external secretions. S-IgA consists of ten polypeptides produced in two different cell lineages. The heavy and light chains in plasma cells assemble into IgA, which on association with J chain become polymerized, whereas secretory component (SC) is added during transport across the epithelium. Recombinant chimeric mouse-human monomeric, dimeric, and S-IgA antibodies have been produced in a single CHO cell sequentially transfected with expression vectors carrying three independent selective markers for chimeric heavy and light chains, human J chain, and human SC, respectively. Biochemical characterization of the various molecular forms indicates that the assembly of the various polypeptides resulted in species of the expected size and covalence. All chimeric IgA antibodies retained the antigen-binding capacity of the parent mouse IgA antibody. The resistance of S-IgA to protease-rich intestinal washes was enhanced when compared with dimeric IgA lacking associated SC. Up to 20 micrograms of recombinant S-IgA per 1 x 10(6) cells were recovered in 24 h with the best producing clones. We conclude that CHO cells programmed de novo with four different genetic elements can assemble functional chimeric S-IgA.
Figures
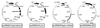



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

