Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes
- PMID: 10077636
- PMCID: PMC15894
- DOI: 10.1073/pnas.96.6.3059
Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes
Abstract
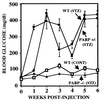
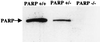
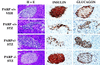
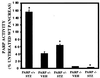
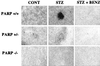
Streptozotocin (STZ) selectively destroys insulin-producing beta islet cells of the pancreas providing a model of type I diabetes. Poly(ADP-ribose) polymerase (PARP) is a nuclear enzyme whose overactivation by DNA strand breaks depletes its substrate NAD+ and then ATP, leading to cellular death from energy depletion. We demonstrate DNA damage and a major activation of PARP in pancreatic islets of STZ-treated mice. These mice display a 500% increase in blood glucose and major pancreatic islet damage. In mice with homozygous targeted deletion of PARP (PARP -/-), blood glucose and pancreatic islet structure are normal, indicating virtually total protection from STZ diabetes. Partial protection occurs in PARP +/- animals. Thus, PARP activation may participate in the pathophysiology of type I diabetes, for which PARP inhibitors might afford therapeutic benefit.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

