Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53
- PMID: 10077639
- PMCID: PMC15897
- DOI: 10.1073/pnas.96.6.3077
Nucleocytoplasmic shuttling of oncoprotein Hdm2 is required for Hdm2-mediated degradation of p53
Abstract
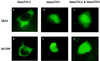
The Hdm2 oncoprotein inhibits p53 functions by two means: (i) it blocks p53's transactivation activity and (ii) it targets p53 for degradation in a proteasome-dependent manner. Recent data indicate that Hdm2 shuttles between the nucleus and the cytoplasm and that the regulation of p53 levels by Hdm2 requires its nuclear export activity. Two different models are consistent with these observations. In the first, Hdm2 binds to p53 in the nucleus and shuttles p53 from the nucleus to the cytoplasm, and then it targets p53 to the cytoplasmic proteasome. Alternatively, Hdm2 and p53 could be exported separately from the nucleus and then associate in the cytoplasm, where Hdm2 promotes the degradation of p53. To distinguish between these two models, several Hdm2 mutants were employed. Hdm2NLS lacks the ability to enter the nucleus, whereas Hdm2NES is deficient in nuclear export. Hdm2NLS, Hdm2NES, or the combination of both mutants were unable to promote p53 degradation in the cotransfected 2KO cells (which were null for both the p53 and mdm2 genes), although wild-type Hdm2 efficiently reduced p53 levels under the same conditions. This observation is not a result of the differences in expression levels or stability between Hdm2 and these mutants. Moreover, coexpression of these mutants had no effect on wild-type Hdm-2-induced p53 destabilization. Thus, Hdm2 must shuttle p53 from the nucleus to the cytoplasm to target it for degradation in the cytoplasm.
Figures



References
-
- Ko L J, Prives C. Genes Dev. 1996;10:1054–1072. - PubMed
-
- Levine A J. Cell. 1997;88:323–331. - PubMed
-
- El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. Cell. 1993;75:817–825. - PubMed
-
- Harper J W, Adami G R, Wei N, Keyomarsi K, Elledge S J. Cell. 1993;75:805–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

