Inhibition of growth, production of insulin-like growth factor-II (IGF-II), and expression of IGF-II mRNA of human cancer cell lines by antagonistic analogs of growth hormone-releasing hormone in vitro
- PMID: 10077643
- PMCID: PMC15901
- DOI: 10.1073/pnas.96.6.3098
Inhibition of growth, production of insulin-like growth factor-II (IGF-II), and expression of IGF-II mRNA of human cancer cell lines by antagonistic analogs of growth hormone-releasing hormone in vitro
Abstract
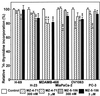
Antagonistic analogs of growth hormone-releasing hormone (GHRH) suppress growth of various tumors in vivo. This effect is exerted in part through inhibition of the GHRH-GH-insulin-like growth factor (IGF)-I axis. Nevertheless, because autocrine/paracrine control of proliferation by IGF-II also is a major factor in many tumors, the interference with this growth-stimulating pathway would offer another approach to tumor control. We thus investigated whether GHRH antagonists MZ-4-71 and MZ-5-156 also act on the tumor cells directly by blocking the production of IGF-II. An increase in the IGF-II concentration in the media during culture was found in 13 of 26 human cancer cell lines tested. Reverse transcription-PCR studies on 8 of these cell lines showed that they also expressed IGF-II mRNA. Antagonists of GHRH significantly inhibited the rate of proliferation of mammary (MDA-MB-468 and ZR-75-1), prostatic (PC-3 and DU-145), and pancreatic (MiaPaCa-2, SW-1990, and Capan-2) cancer cell lines as shown by colorimetric and [3H]thymidine incorporation tests and reduced the expression of IGF-II mRNA in the cells and the concentration of IGF-II secreted into the culture medium. Growth and IGF-II production of lung (H-23 and H-69) and ovarian (OV-1063) cancer cells that express mRNA for IGF-II and excrete large quantities of IGF-II also was marginally suppressed by the antagonists. These findings suggest that antagonistic analogs of GHRH can inhibit growth of certain tumors not only by inhibiting the GHRH-GH-IGF-I axis, but also by reducing the IGF-II production and by interfering with the autocrine regulatory pathway.
Figures



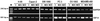
References
-
- Toretsky J A, Helman L J. J Endocrinol. 1996;149:367–372. - PubMed
-
- Schally A V, Kovacs M, Toth K, Comaru-Schally A M. In: Growth Hormone Secretagogues in Clinical Practice. Bercu B B, Walker R F, editors. New York: Dekker; 1998. pp. 145–162.
-
- Daughaday W H. Endocrinology. 1990;127:1–4. - PubMed
-
- Pollak M N, Schally A V. Proc Soc Exp Biol Med. 1998;217:143–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

