Transdermal photopolymerization for minimally invasive implantation
- PMID: 10077644
- PMCID: PMC15902
- DOI: 10.1073/pnas.96.6.3104
Transdermal photopolymerization for minimally invasive implantation
Abstract
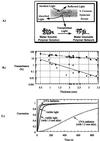
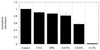
Photopolymerizations are widely used in medicine to create polymer networks for use in applications such as bone restorations and coatings for artificial implants. These photopolymerizations occur by directly exposing materials to light in "open" environments such as the oral cavity or during invasive procedures such as surgery. We hypothesized that light, which penetrates tissue including skin, could cause a photopolymerization indirectly. Liquid materials then could be injected s.c. and solidified by exposing the exterior surface of the skin to light. To test this hypothesis, the penetration of UVA and visible light through skin was studied. Modeling predicted the feasibility of transdermal polymerization with only 2 min of light exposure required to photopolymerize an implant underneath human skin. To establish the validity of these modeling studies, transdermal photopolymerization first was applied to tissue engineering by using "injectable" cartilage as a model system. Polymer/chondrocyte constructs were injected s.c. and transdermally photopolymerized. Implants harvested at 2, 4, and 7 weeks demonstrated collagen and proteoglycan production and histology with tissue structure comparable to native neocartilage. To further examine this phenomenon and test the applicability of transdermal photopolymerization for drug release devices, albumin, a model protein, was released for 1 week from photopolymerized hydrogels. With further study, transdermal photpolymerization potentially could be used to create a variety of new, minimally invasive surgical procedures in applications ranging from plastic and orthopedic surgery to tissue engineering and drug delivery.
Figures

References
-
- Hill-West J, Dunn R, Hubbell J. J Surg Res. 1995;59:759–763. - PubMed
-
- Sawhney A, Lyman F, Yao F, Levine M, Jarrett P. Proc Int Symp Controlled Release Bioact Mater. 1996;23:236–237.
-
- Watts D C. In: Materials Science and Technology: A Comprehensive Treatment. Williams D F, editor. Vol. 14. New York: VCH; 1992. pp. 209–258.
-
- Peppas N. Hydrogels in Medicine and Pharmacy. II. Boca Raton, FL: CRC; 1987.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

