Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1
- PMID: 10077651
- PMCID: PMC15909
- DOI: 10.1073/pnas.96.6.3143
Mechanism of increased iron absorption in murine model of hereditary hemochromatosis: increased duodenal expression of the iron transporter DMT1
Abstract
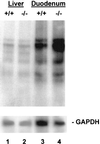
Hereditary hemochromatosis (HH) is a common autosomal recessive disorder characterized by tissue iron deposition secondary to excessive dietary iron absorption. We recently reported that HFE, the protein defective in HH, was physically associated with the transferrin receptor (TfR) in duodenal crypt cells and proposed that mutations in HFE attenuate the uptake of transferrin-bound iron from plasma by duodenal crypt cells, leading to up-regulation of transporters for dietary iron. Here, we tested the hypothesis that HFE-/- mice have increased duodenal expression of the divalent metal transporter (DMT1). By 4 weeks of age, the HFE-/- mice demonstrated iron loading when compared with HFE+/+ littermates, with elevated transferrin saturations (68.4% vs. 49.8%) and elevated liver iron concentrations (985 micrograms vs. 381 micrograms). By using Northern blot analyses, we quantitated duodenal expression of both classes of DMT1 transcripts: one containing an iron responsive element (IRE), called DMT1(IRE), and one containing no IRE, called DMT1(non-IRE). The positive control for DMT1 up-regulation was a murine model of dietary iron deficiency that demonstrated greatly increased levels of duodenal DMT1(IRE) mRNA. HFE-/- mice also demonstrated an increase in duodenal DMT1(IRE) mRNA (average 7.7-fold), despite their elevated transferrin saturation and hepatic iron content. Duodenal expression of DMT1(non-IRE) was not increased, nor was hepatic expression of DMT1 increased. These data support the model for HH in which HFE mutations lead to inappropriately low crypt cell iron, with resultant stabilization of DMT1(IRE) mRNA, up-regulation of DMT1, and increased absorption of dietary iron.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

